Nuclear physics is the area of science that studies the structure of atomic nuclei and the transformations that nuclei undergo. It is important to have a good understanding of the nucleus to have a coherent picture of matter and energy. Furthermore, nuclear physics has given rise to a host of technologies in medical science and life science research.
You must know your way around this topic for the new MCAT. Nuclear physics is crucial to interventional and diagnostic radiology as well as to biochemistry and molecular biology, for without the use of radiolabeling techniques it would be difficult to imagine how many discoveries would have been possible.
WikiPremed Resources
Nuclear Physics Cards
Conceptual Vocabulary Self-Test
Basic Terms Crossword Puzzle
Basic Puzzle Solution
Conceptual Vocabulary for Nuclear Physics
Nuclear Physics
Nuclear physics is the branch of physics concerned with the nucleus of the atom.
Radioactive decay is the process in which an unstable atomic nucleus loses energy by emitting radiation in the form of particles or electromagnetic waves.
Radiation in nuclear physics describes energy in the form of waves or moving subatomic particles.
Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus.
Nuclear fission is the splitting of the nucleus of an atom into lighter nuclei often producing photons in the form of gamma rays, free neutrons and other subatomic particles as by-products.
Nuclear fusion is the process by which multiple atomic particles join together to form a heavier nucleus.
In nuclear physics, beta decay is a type of radioactive decay in which an electron or a positron is emitted.
Beta particles are high-energy, high-speed electrons or positrons emitted by certain types of radioactive nuclei.
Alpha decay is a type of radioactive decay in which an atomic nucleus emits two protons and two neutrons bound together into a particle identical to a helium nucleus.
Gamma rays are forms of electromagnetic radiation of a specific frequency produced from sub-atomic particle interaction, such as electron-positron annihilation and radioactive decay.
The half-life of a quantity, subject to exponential decay, is the time required for the quantity to decay to half of its initial value.
Deuterium, also called heavy hydrogen, is a stable isotope of hydrogen with a natural abundance in the oceans of Earth of approximately one atom in 6500 of hydrogen.
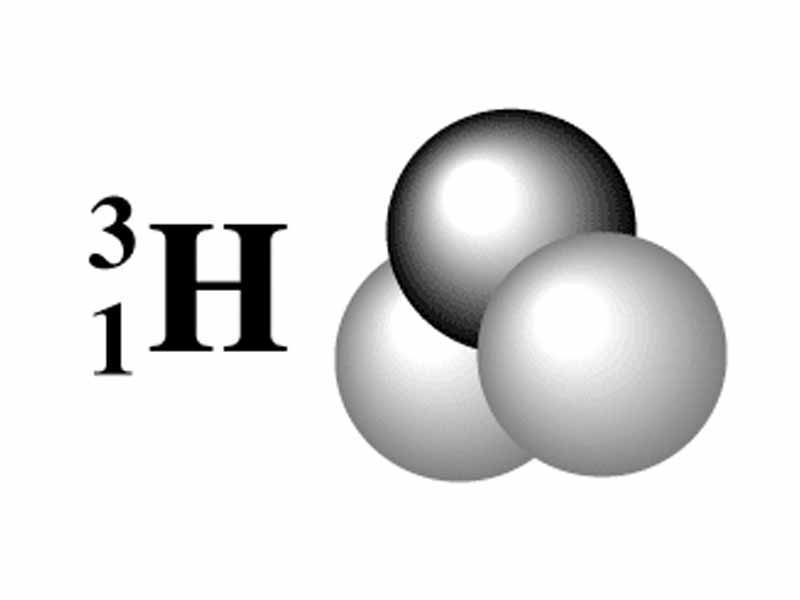
Tritium is a radioactive isotope of hydrogen with a nucleus containing one proton and two neutrons.
Antineutrinos, the antiparticles of neutrinos, are neutral particles produced in nuclear beta decay.
Electron capture, sometimes called inverse beta decay, is a decay mode for isotopes that will occur when there are too many protons in the nucleus of an atom and insufficient energy to emit a positron.
A quantity is said to be subject to exponential decay if it decreases at a rate proportional to its value.
Carbon-14 is a radioactive isotope of carbon. Its nucleus contains 6 protons and 8 neutrons.
Nuclear transmutation is the conversion of one chemical element or isotope into another, which occurs through nuclear reactions.
Uranium-235 is an isotope of uranium that differs from the element's other common isotope, uranium-238, by its ability to cause a rapidly expanding fission chain reaction, i.e., it is fissile.
Enriched uranium is a sample of uranium in which the percent composition of uranium-235 has been increased through the process of isotope separation.
The nuclear force or residual strong force is the force between two or more nucleons. It is responsible for binding of protons and neutrons into atomic nuclei.
The becquerel is the SI derived unit of radioactivity equal to one nucleus decay per second.
Positron emission is a type of beta decay, sometimes referred to as beta plus decay.
Proton emission, also known as proton radioactivity, is a type of radioactive decay in which a proton is ejected from a nucleus.
Neutron emission is a type of radioactive decay in which an atom contains excess neutrons and a neutron is simply ejected from the nucleus.
Heavy water is water which contains a higher proportion than normal of the isotope deuterium as deuterium oxideor as deuterium protium oxide.
Nucleosynthesis is the process of creating new atomic nuclei from preexisting nucleons.
A critical mass is the smallest amount of fissile material needed for a sustained nuclear chain reaction.
A control rod is a rod made of chemical elements capable of absorbing many neutrons without fissioning themselves. They are used in nuclear reactors to affect the rate of fission.
A nucleon is a collective name for two baryons: the neutron and the proton.
The strong interaction is today understood to represent the interactions between quarks and gluons as detailed by the theory of quantum chromodynamics.
The curie is a unit of radioactivity which is roughly the activity of 1 gram of the radium isotope 226Ra.
In nuclear engineering, a neutron moderator is a medium which reduces the velocity of fast neutrons, thereby turning them into thermal neutrons capable of sustaining a nuclear chain reaction.
In nuclear engineering, a fissile material is one that is capable of sustaining a chain reaction of nuclear fission.
Plutonium-239 is an isotope of plutonium. It is one of the three fissile isotopes used for the production of nuclear weapons and in nuclear reactors as a source of energy, the others being uranium-235 and uranium-233.
In nuclear physics, a magic number is a number of nucleons, either protons or neutrons, such that they are arranged into complete shells within the atomic nucleus.
The island of stability is a term from nuclear physics that describes the possibility of elements with particularly stable magic numbers of protons and neutrons.
The decay chain refers to the radioactive decay of different discrete radioactive decay products as a sequential series of transformations.
The neutron temperature, also called the neutron energy, indicates a free neutron's kinetic energy, usually given in electron volts.
Stellar nucleosynthesis is the collective term for the nuclear reactions taking place in stars to build the nuclei of the heavier elements.
A nuclear reaction is a process in which two nuclei or nuclear particles collide to produce products different from the initial particles.
A breeder reactor is a nuclear reactor that consumes fissile and fertile material at the same time as it creates new fissile material.
The liquid drop model is a model in nuclear physics which treats the nucleus as a drop of incompressible nuclear fluid made of nucleons held together by the strong nuclear force.
The nuclear shell model is a model of the atomic nucleus which uses the Pauli principle to describe the structure of the nucleus in terms of energy levels.
Cluster decay is the nuclear process in which a radioactive atom emits an array of neutrons and protons.
Isomeric transition is a radioactive decay process that occurs where a nucleus in an excited meta state emits a gamma ray, which returns the nucleus to the ground state.
A free neutron is a neutron that exists outside of an atomic nucleus.
Big Bang nucleosynthesis refers to the production of nuclei other than those of the normal, light isotope of hydrogen during the early phases of the universe.
Cosmic ray spallation is a form of naturally occurring nuclear fission and nucleosynthesis due to the impact of cosmic rays on an object.
The semi-empirical mass formula is used to approximate the mass and various other properties of an atomic nucleus.
The Auger effect is a phenomenon in physics in which the emission of an electron from an atom causes the emission of a second electron.
Internal conversion is a decay process where an excited nucleus interacts with an electron in one of the lower electron shells, causing the electron to be emitted from the atom, appearing to be a classical beta particle, though without beta decay taking place.
A prompt neutron is a neutron immediately emitted by a nuclear fission event, as opposed to a delayed neutron which is emitted by one of the fission products anytime from a few milliseconds to a few minutes later.
A nuclear reactor assembly is prompt critical when, for each nuclear fission event, one or more of the prompt neutrons released causes an additional fission event.
A tokamak is a machine producing a toroidal magnetic field for confining a plasma.
Magnetic confinement fusion is an approach to generating fusion energy that uses magnetic fields to confine the fusion fuel in the form of a plasma.
Fertile material is a term used to describe nuclides which generally themselves do not undergo induced fission but from which fissile material is generated by neutron absorption and subsequent nuclei conversions.
The interacting boson model is a model in nuclear physics in which nucleons pair up, essentially acting as a single particle with boson properties, with integral spin of 0, 2 or 4.
Woods Saxon potential is a mean field potential for the nucleons inside the atomic nucleus, which is used to approximately describe the forces applied on each nucleon, in the shell model for the structure of the nucleus.
The triple-alpha process is the process by which three helium nuclei are transformed into carbon in stellar interiors having a high helium abundance.
The carbon burning process is a nuclear fusion reaction that occurs in massive stars that have used up the lighter elements in their cores.
The Lawson criterion is an important general measure of a system that defines the conditions needed for a fusion reactor to reach ignition
Inertial confinement fusion is a process where nuclear fusion reactions are initiated by heating and compressing a fuel target, typically in the form of a pellet containing a mixture of deuterium and tritium.
The Z- or zeta pinch is a type of plasma confinement system that uses an electrical current in the plasma to generate a magnetic field that compresses it.
A reversed-field pinch is a toroidal pinch which uses a unique magnetic field configuration as a scheme to magnetically confine a plasma, primarily to study magnetic fusion energy.
A Field-Reversed Configuration is a device developed for magnetic fusion energy research that confines a plasma on closed magnetic field lines without a central penetration.
A levitated dipole is a unique form of fusion reactor technology using a solid superconducting torus, magnetically levitated in the reactor chamber.