The periodic table is an arrangement of the chemical elements ordered by atomic number in columns and rows (groups and periods). The design of the periodic table reflects the observation that many properties of the chemical elements are periodic functions of their atomic number. The periodic table is arranged so as to emphasize these periodic properties. In concert with an atom's valence shell configuration, the periodic properties of an element provide you with a thumbnail picture of its tendencies, allowing you to predict how it will behave in different contexts. The periodic properties are atomic radius, ionization energy, electron affinity and electronegativity.
Only rarely to questions appear on the MCAT that ask you directly about periodic properties, but like so many of these early chapters, the material is fundamental to much else which comes later. Of the periodic properties, the electronegativity is especially crucial, although all of them are important. Considering the electronegativity difference between bonded atoms is the first step to assigning bond polarity. Understanding bond polarity is necessary for predicting intermolecular forces, solubility relationships or assigning oxidation numbers, for example.
WikiPremed Resources
Periodic Table
Conceptual Vocabulary Self-Test
Basic Terms Crossword Puzzle
Basic Puzzle Solution
Conceptual Vocabulary for Periodic Trends
Periodic Trends
The periodic table of the chemical elements is a tabular method of displaying the chemical elements credited to Russian chemist Dmitri Mendeleev in 1869.
A chemical element is a type of atom that is defined by its atomic number; that is, by the number of protons in its nucleus.
The atomic number or proton number is the number of protons found in the nucleus of an atom.
A group, also known as a family, is a vertical column in the periodic table of the chemical elements.
Electronegativity is a chemical property which describes the power of an atom to attract electrons towards itself.
The ionization energy of an atom or molecule is the energy required to remove one mole of electrons from one mole of isolated gaseous atoms or ions.
A chemical symbol is an abbreviation or short representation of the name of a chemical element, generally assigned in relation to its Latin name.
The noble gases are the elements in group 18 of the periodic table. It is also called helium family or neon family.
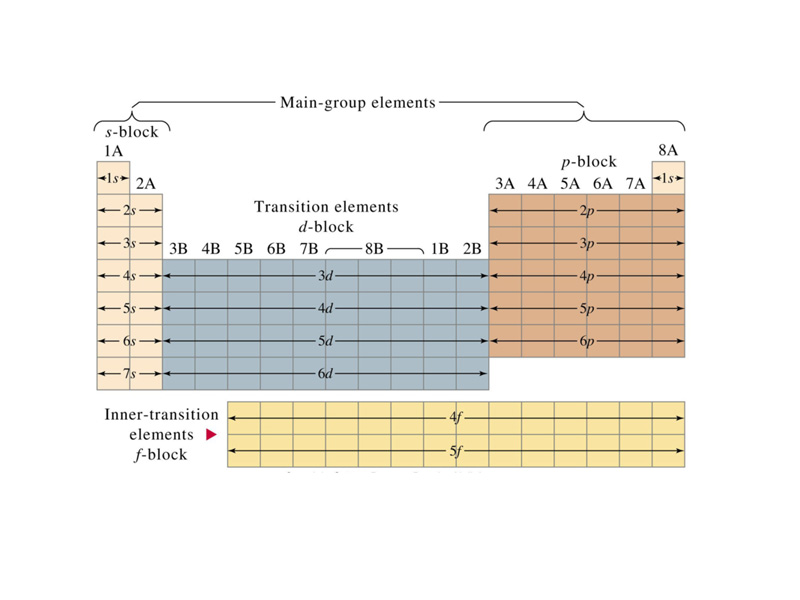
The term transition metal commonly refers to any element in the d-block of the periodic table, including zinc, cadmium and mercury. This corresponds to groups 3 to 12 on the periodic table.
The electron affinity of an atom or molecule is the energy required to detach an electron from a singly charged negative ion or, inversely, the energy released when an electron is attached to a neutral atom or molecule.
Dimitri Mendeleev (1834 - 1907) was a Russian chemist credited as being the primary creator of the first version of the periodic table of elements.
The atomic mass is the mass of an atom at rest.
The alkali metals are a series of elements comprising Group 1 of the periodic table: lithium (Li), sodium (Na), potassium (K), rubidium (Rb), caesium (Cs), and francium (Fr).
The alkaline earth metals are a series of elements comprising Group 2 of the periodic table: beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba) and radium (Ra).
The halogens are a series of nonmetal elements from Group 17 of the periodic table, comprising fluorine, F; chlorine, Cl; bromine, Br; iodine, I; and astatine, At.
The mass number is the number of nucleons in an atomic nucleus.
The unified atomic mass unit (u), or dalton (Da), is defined to be one twelfth of the mass of an unbound atom of the carbon-12 nuclide, at rest and in its ground state.
The s-block of the periodic table of elements consists of the first two groups: the alkali metals and alkaline earth metals, plus hydrogen and helium.
The p-block of the periodic table of the elements consists of the last six groups minus helium.
The somewhat hazily defined physical property known as atomic radius represents a reasonable attempt to quantify the size of atoms and ions, based both on experimental measurements and calculational methods.
The d-block of the periodic table of the elements consists of those periodic table groups that contain elements in which, in the atomic ground state, the highest-energy electron is in a d-orbital.
The lanthanide series comprises the 15 elements with atomic numbers 57 through 71, from lanthanum to lutetium.
The actinide series encompasses the 15 chemical elements that lie between actinium and lawrencium on the periodic table, with atomic numbers 89 - 103.
Rare earth elements and metals are a collection of sixteen chemical elements in the periodic table, namely scandium, yttrium, and fourteen of the fifteen lanthanoids (excluding promethium), which naturally occur on the Earth.
The f-block of the periodic table of the elements consists of those elements (sometimes referred to as the inner transition elements) for which, in the atomic ground state, the highest-energy electrons occupy f-orbitals.
The chalcogens are the name for the periodic table group 16 in the periodic table. It is sometimes known as the oxygen family.