Atomic Theory is the branch of chemistry concerned with the smallest form of an element that can exist chemically, the atom. Classical physics is helpful to understanding some properties of atoms. However, the range of behaviors of atoms exceeds the descriptive powers of classical physics. To explain the line spectrum of hydrogen, for example, Neils Bohr develped his early form of atomic theory. A more complete picture of the electronic structure of the atom is provided by modern quantum electrodynamics.
Questions directly concerned with Atomic Theory, or more generally, basic quantum mechanics, do appear with fair regularity on the MCAT, although they tend to be easier questions than they may seem at first glance. More important than the direct appearance of these concepts on the exam is that these initial chapters of Chemistry, dealing with the instrinsic structure of matter, i.e. Atomic Theory, Periodic Properties, and Chemical Bonding, are absolutely crucial for the scientific understanding of the physical and natural world. The rest of General Chemistry, Organic Chemistry, and Biology will make profoundly better sense, and be much more interesting besides, if you take special care to understand the structure of matter.
WikiPremed Resources
Atomic Theory Concepts
Conceptual Vocabulary Self-Test
Basic Terms Crossword Puzzle
Basic Puzzle Solution
Conceptual Vocabulary for Atomic Theory
Atomic Theory
An atom is the smallest particle still characterizing a chemical element
The electron is a fundamental subatomic particle that carries a negative electric charge.
The proton is a subatomic particle with an electric charge of one positive fundamental unit, a diameter of about 1.5 fm femtometer, and a mass that is about 1836 times the mass of an electron.
The neutron is a subatomic particle with no net electric charge and a mass that is slightly more than a proton
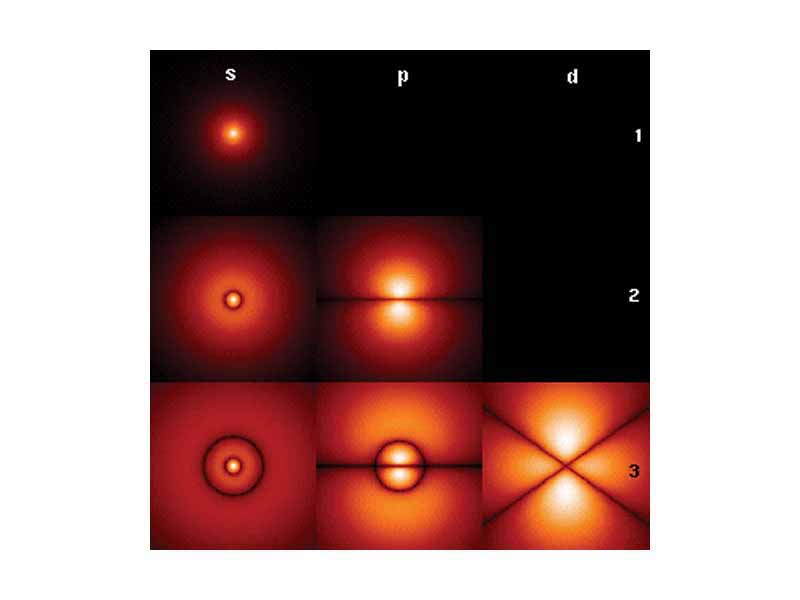
An atomic orbital is a mathematical description of the region in which an electron may be found around a single atom.
An ion is an atom or molecule which has lost or gained one or more electrons, making it negatively or positively charged.
Isotopes are any of the several different forms of an element with nuclei having the same number of protons but different numbers of neutrons.
The Bohr model depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus.
Hydrogen is a chemical element represented by the symbol H and an atomic number of 1.
Valence electrons are the electrons contained in the outermost electron shell of an atom.
The electron configuration is the arrangement of electrons in an atom, molecule, or other physical structure such as a crystal.
An electron shell, also known as a main energy level, is a group of atomic orbitals with the same value of the principal quantum number.
Ernest Rutherford was a nuclear physicist who pioneered the orbital theory of the atom through his discovery of scattering off the nucleus with his gold foil experiment.
Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus.
A quantum leap is a change of an electron from one energy state to another within an atom.
An element's emission spectrum is the relative intensity of electromagnetic radiation of each frequency it emits when it is excited.
Spin is the angular momentum intrinsic to a body, as opposed to orbital angular momentum, which is the motion of its center of mass about an external point.
Hund's rules are a simple set of rules used to determine the term symbol that corresponds to the ground state of a multi-electron atom.
The Aufbau is used to determine the electron configuration of an atom, molecule or ion, postulating a hypothetical process in which an atom is built up by progressively adding electrons.
The law of definite proportions states that a chemical compound always contains exactly the same proportion of elements by mass.
The Rutherford model showed that the plum pudding model of the atom of J. J. Thomson was incorrect, presenting the atom as containing a central charge concentrated into a very small volume in comparison to the rest of the atom.
Quantum mechanics is the study of the relationship between energy quanta and matter, in particular between photons and valence shell electrons.
The Heisenberg uncertainty principle gives a lower bound on the product of the standard deviations of position and momentum for a system, implying that it is impossible to have a particle that has an arbitrarily well-defined position and momentum simultaneously.
The quantum state of a system corresponds to a set of numbers that fully describe a quantum system.
The Pauli exclusion principle explains why matter occupies space exclusively for itself and does not allow other material objects to pass through it, while at the same time allowing light and radiation to pass.
An excited state of a system is any quantum state of the system that has a higher energy than the ground state.
Observation of the phenomenon of Rutherford scattering of alpha particles incident on gold foil led to the development of the orbital theory of the atom.
The principal quantum number has the greatest correlation to energy of the quantum numbers describing the unique quantum state of an electron in an atom.
A black body is an object that absorbs all electromagnetic radiation that falls onto it. No radiation passes through it and none is reflected.
Cathode rays are streams of electrons observed in vacuum tubes.
The purpose of Robert Millikan and Harvey Fletcher's oil-drop experiment (1909) was to measure the electric charge of the electron.
Sir Joseph John Thomson (1856 - 1940) was a British scientist credited for the discovery of the electron, of isotopes, and the invention of the mass spectrometer.
The spin quantum number is a quantum number that parametrizes the intrinsic angular momentum of a given particle.
The magnetic quantum number, along with the principal quantum number, the azimuthal quantum number, and the spin quantum number, describes the unique quantum state of an electron.
The Schrödinger equation describes the space- and time- dependence of quantum mechanical systems.
A spectral line is a dark or bright line in an otherwise uniform and continuous spectrum, resulting from an excess or deficiency of photons in a narrow frequency range.
Niels Bohr (1885 - 1962) was a Danish physicist who made fundamental contributions to understanding atomic structure and quantum mechanics, for which he received the Nobel Prize in 1922.
The plum pudding model of the atom was proposed by J. J. Thomson, the discoverer of the electron in 1897 before the discovery of the atomic nucleus.
The Azimuthal quantum number (or orbital angular momentum quantum number) is the quantum number for an atomic orbital which determines its orbital angular momentum.
The Balmer series describes a series of spectral line emissions of the hydrogen atom that reflect emissions of photons by electrons in excited states transitioning to the quantum level described by the principal quantum number n equals 2.
John Dalton (1766 - 1844) was an English chemist, meteorologist and physicist, best known for his pioneering work in the development of modern atomic theory.
In the electron cloud analogy, the probability density of an electron, or wavefunction, is described as a region of space around the atomic or molecular nucleus representing the electron's likely location.
A stationary state is an eigenstate of a Hamiltonian, or in other words, a state of definite energy. The corresponding probability density has no time dependence.
The Lyman series is the series of transitions and resulting emission lines of the hydrogen atom as an electron goes from an electron shell of principal quantum number greater than or equal to 2 to the ground state.
The Rydberg formula is used in atomic physics for describing the wavelengths of spectral lines of many chemical elements.
The Stark effect is the shifting and splitting of spectral lines of atoms and molecules due to the presence of an external static electric field.
The Zeeman effect is the splitting of a spectral line into several components in the presence of a static magnetic field.
The Paschen series is the series of transitions and resulting emission lines of the hydrogen atom as an electron goes from an electron shell greater than or equal to 4 to n = 3.
Hyperfine structure is a small perturbation in the energy levels, or spectra, of atoms or molecules due to the magnetic dipole-dipole interaction, arising from the interaction of the nuclear magnetic moment with the magnetic field of the electron.
In atomic physics, the Brackett series describes a series of spectral line emissions of the hydrogen atom that appear in emission when hydrogen atoms' electrons descend to the fourth energy level from a higher level.
In 1914, the Franck-Hertz experiment elegantly supported Niels Bohr's model of the atom by demonstrating that atoms could indeed only absorb specific amounts of energy.
Moseley's law is an empirical law concerning the characteristic x-rays that are emitted by atoms which justified the conception of the nuclear model of the atom.
In X-ray spectroscopy, K-alpha emission lines result when an electron transitions to the innermost K shell from a 2p orbital of the second or L shell.
The spin-orbit interaction is any interaction of a particle's spin with its motion.
The Siegbahn notation is used in x-ray spectroscopy to name the spectral lines that are characteristic to elements. It was created by Manne Siegbahn.