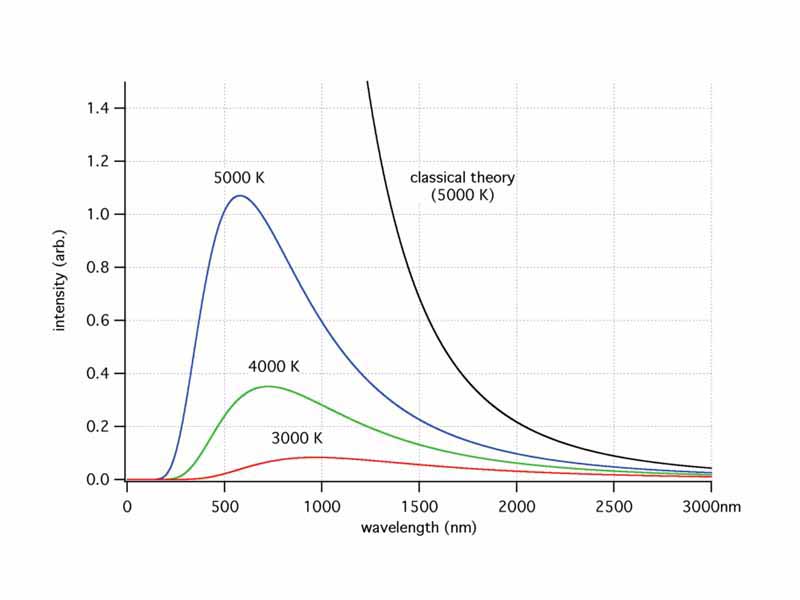
Thermal radiation is emitted by an object at any temperature above absolute zero. The classical perspective on thermal radiation presents a model of charged particles accelerating (vibrating) and emitting electromagnetic waves similarly to the charges of a broadcast antenna. The breakdown in the classical model occurred in that the distribution of observed radiation emitted by a black body at short wave lengths, a contradiction known as the ultraviolet catastrophe. Planck's theory contained two breakthrough postulates which were the starting points of quantum theory, firstly, that the energy of vibrating molecules were quantized, possessing discrete states of energy or quantum states, and secondly, that the light energy absorbed or emitted was quantized. That light consists of discrete units of energy, quanta, or photons. From this work Planck derived his famous expression of photon energy: E = hf
A basic understanding the quantum nature of light is essential to understanding the early history of Atomic Theory. Without Planck's work, which demonstrated the quantized nature of light, Bohr would not have possessed the analytical framework to interpret the line spectrum of hydrogen. Planck's work introduced the particle/wave model of light that characterizes modern quantum electrodynamics.
A basic understanding the quantum nature of light is essential to understanding the early history of Atomic Theory. Without Planck's work, which demonstrated the quantized nature of light, Bohr would not have possessed the analytical framework to interpret the line spectrum of hydrogen. Planck's work introduced the particle/wave model of light that characterizes modern quantum electrodynamics.