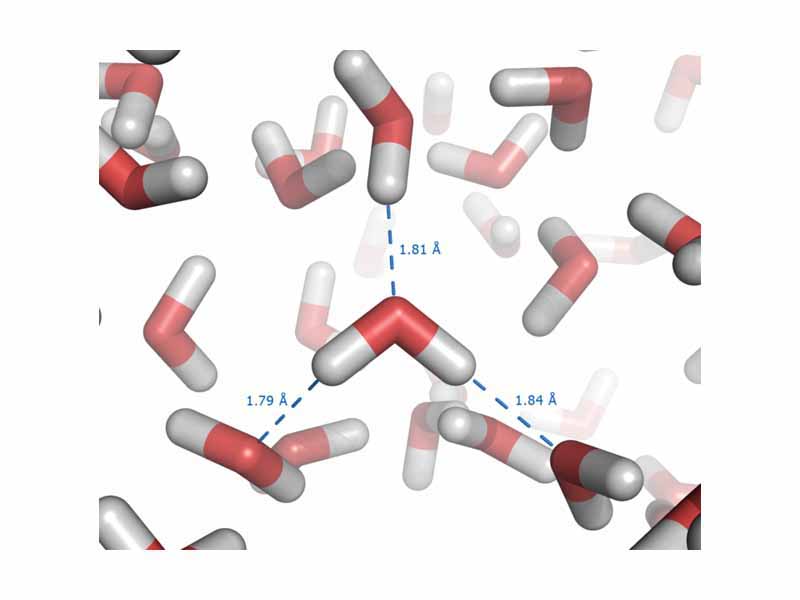
Intermolecular forces occur between molecules. Although intermolecular forces are weaker than chemical bonds, they are very significant in determining the physical and chemical properties of substances. There are three principal types of intermolecular force: 1) dipole-dipole forces, which occur between polar molecules 2) London dispersion forces, which occcur due to small instantaneous dipoles and 3) hydrogen bonding, which occur between molecules in which hydrogen is bonded to a small electronegative atom such as oxygen. Because the polarity of such molecules is occupied on one end by a bare hydrogen proton, without inner shells of electrons to shield it, hydrogen bonding gives rise to intermolecular forces which are significantly stronger than any other type.
Because intermolecular forces are of great importance in living organisms, this topic is of major MCAT importance. The MCAT will often pose both explicit questions from this topic and questions depending on reasoning which takes intermolecular force as its starting point. Furthermore, many other topics, such as States of Matter or Solutions in Chemistry or Proteins or Membranes in Biology greatly rely on your understanding of Intermolecular Forces.
WikiPremed Resources
Intermolecular Forces Images
Conceptual Vocabulary Self-Test
Basic Terms Crossword Puzzle
Basic Puzzle Solution
Conceptual Vocabulary for Intermolecular Force
Intermolecular Force
An intermolecular force is a force that acts between stable molecules or between functional groups of macromolecules which are generally much weaker than the chemical bonding forces.
A hydrogen bond is a special type of dipole-dipole interaction that exists between an electronegative atom and a hydrogen atom bonded to another electronegative atom.
Hydrophobicity refers to the physical property of a molecule that is repelled from a mass of water
The name van der Waals force is sometimes used as a synonym for the totality of non-covalent forces which act between stable molecules.
The water dimer consists of two water molecules loosely bound by a hydrogen bond. It is the smallest water cluster.
A noncovalent bond is a type of chemical bond, typically between macromolecules, that does not involve the sharing of pairs of electrons, but rather involves more dispersed variations of electromagnetic interactions.
The debye (symbol: D) is a non-SI and non-CGS unit of electrical dipole moment.
The Lennard-Jones potential is a simple mathematical model that represents the condition of neutral atoms and molecules being subject to an attractive force at long ranges and a repulsive force at short ranges.