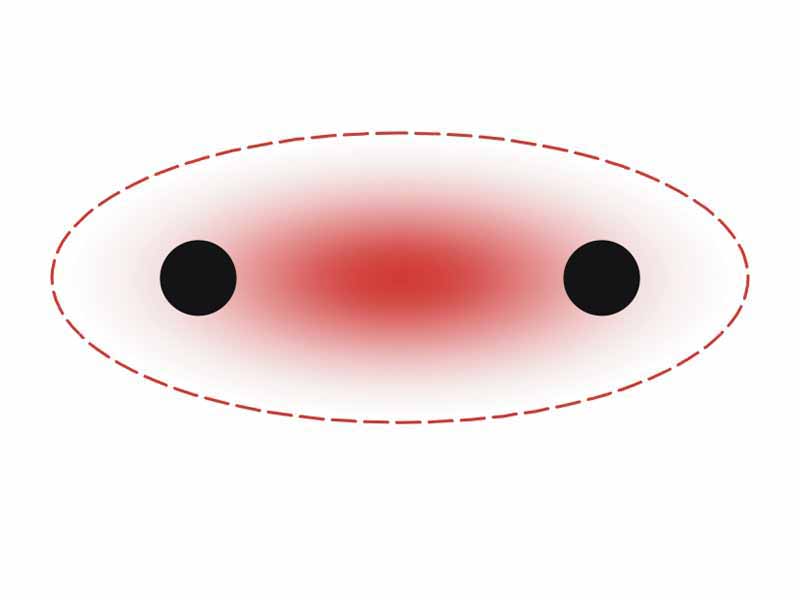
Chemical bond is a term used to describe the range of different types of attractions that can occur to allow two or more atoms to become persistently associated and take on properties as a group. Although there aren't clear distinctions at the boundaries, there are basically three types of chemical bonds: metallic, ionic, and covalent. In pure metals and many alloys, the outermost s and p electrons become freely mobile, forming a kind of electron 'fluid'. Metallic bonding describes the attractions between the lattice of positively charged metal ions and the surrounding mobile electrons. Ionding bonding results from the transfer of electrons from one species to another. The bond is the resulting attraction between the cation and anion formed. Covalent bonds result when atoms share a pair of electrons. The bond consists of the attraction of the electron pair in the internuclear space for the nuclei of the bonded atoms.
Although you will probably see a few traditionally framed chemical bonding questions on the MCAT, no other topic is more important for your overall goals in MCAT preparation in the sciences. Huge portions of the general chemistry, organic chemistry, and biology can only be coherently understood if you understand the fundamentals of chemical bonding.
WikiPremed Resources
Chemical Bonding Concepts
Conceptual Vocabulary Self-Test
Basic Terms Crossword Puzzle
Basic Puzzle Solution
Conceptual Vocabulary for Chemical Bonding
Chemical Bonding
A chemical bond is the physical process responsible for the attractive interactions between atoms and molecules which confers stability to diatomic and polyatomic chemical compounds.
Covalent bonding is a form of attraction-to-repulsion stability that forms between atoms when they share electrons.
A molecule is defined as a sufficiently stable electrically neutral group of at least two atoms in a definite arrangement held together by strong chemical bonds.
A chemical compound is a substance consisting of two or more elements chemically-bonded together in a fixed proportion by mass.
An ionic bond (or electrovalent bond) is a type of chemical bond based on electrostatic forces between two oppositely-charged ions.
The octet rule is a simple chemical rule of thumb that states that atoms tend to combine in such a way that they each have a noble gas configuration in their valence shells.
Bond energy is the enthalpy change involved with breaking up a neutral molecule into subtitutent neutral elements.
Lewis structures, also called electron-dot structures or electron-dot diagrams, are diagrams that show the bonding between atoms of a molecule, and the lone pairs of electrons that may exist in the molecule.
Valence bond theory explains the nature of a chemical bond in a molecule in terms of atomic valencies.
Resonance is a tool used to represent and model certain types of non-classical molecular structures arising when no single conventional model showing electrons shared exclusively by two atoms can actually represent the observed molecule.
Valence is a measure of the number of chemical bonds formed by the atoms of a given element.
Valence electrons are the electrons contained in the outermost electron shell of an atom.
A formal charge is a partial charge on an atom in a molecule assigned by assuming that electrons in a chemical bond are shared equally between atoms, regardless of relative electronegativity.
The bond dipole moment is a measure for the polarity of a chemical bond within a molecule.
Bond dissociation energy is defined as the standard enthalpy change when a bond is cleaved by homolysis at 0K (absolute zero).
Bond order is the number of bonds between a pair of atoms.
Chemical polarity is a concept in chemistry which describes how bonding electrons may or may not be equally shared between atoms.
A molecular orbital is a region in which an electron may be found in a molecule.
Molecular orbital theory is a method for determining molecular structure in which electrons are not assigned to individual bonds between atoms, but are treated as under the influence of the nuclei in the whole molecule.
A lone pair is a valence electron pair without bonding or sharing with other atoms.
Orbital hybridization is the concept of mixing atomic orbitals to form new hybrid orbitals suitable for the qualitative description of atomic bonding properties.
Delocalized electrons are electrons in a molecule that are not associated with a single atom or covalent bond but contained within an orbital that extends over several adjacent atoms.
VSEPR theory is a model in chemistry used for the representation of shapes of individual molecules, based upon their extent of electron-pair electrostatic repulsion.
Sigma bonds are a type of covalent chemical bond which is symmetrical with respect to rotation about the bond axis.
Pi bonds are covalent chemical bonds where two lobes of one involved electron orbital overlap two lobes of the other involved electron orbital with only one of the orbital's nodal planes passing through both of the involved nuclei.
A coordinate covalent bond is a description of covalent bonding between two atoms in which both electrons shared in the bond come from the same atom.
Metallic bonding is the bonding between atoms within metals involving the delocalized sharing of free electrons among a lattice of metal atoms.
Linus Carl Pauling (1901 - 1994) was an American quantum chemist and biochemist who pioneered the application of quantum mechanics to describing the nature of chemical bonds. He also made important contributions to crystal and protein structure determination.
Bent bond is a term in chemistry that refers to a type of covalent chemical bond with a geometry somewhat reminescent of a banana that occurs within small ring molecules, such as cyclopropane.
Delta bonds are chemical bonds of the covalent type where four lobes of one involved electron orbital overlap four lobes of the other involved electron orbital.