At this stage in the MCAT course, we are carefully studying the nature of chemical bonds in themselves. In a few weeks, we'll be studying chemical reactions in which old bonds are broken and new bonds formed. Let us preview an important conceptual cluster, the internal energy change involved in chemical bond formation that will be so important to our understanding of the thermochemistry of reactions later.
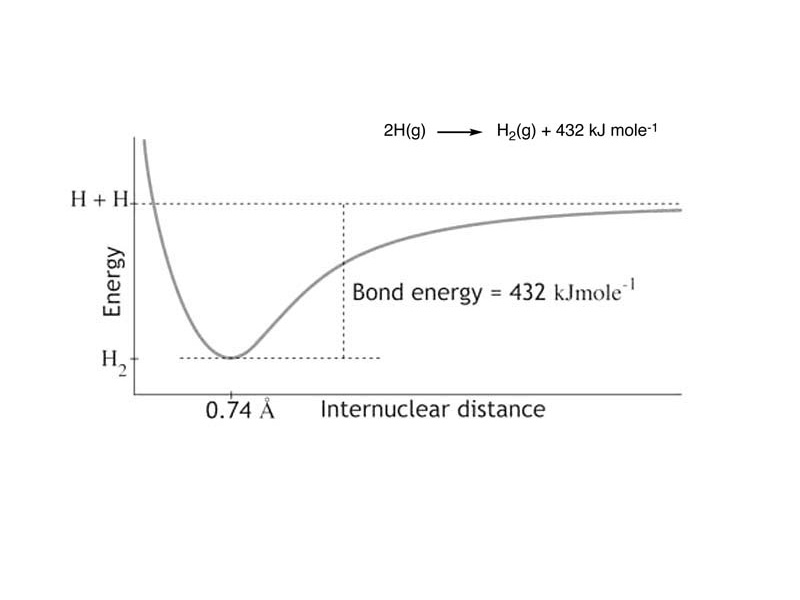
The internal energy change of two atoms undergoing bond formation involves a decrease in electrostatic potential energy. The total energy of the bound molecule, with the atoms separated by the bond distance, is less than the energy of this simple system with the atoms fully separated. Why is this so?
Imagine bond formation. As electrons within nonbonded atoms form molecular orbitals, the nuclei are drawn inward along the bond axis and internal energy decreases. Try to picture it in your mind. As the bonding electrons form molecular orbitals, their presence within the internuclear space draws the nuclei inward. As the negatively charged electrons in the bonding orbital close distance with the positively charged nuclei on either side, unlike charges are drawing nearer to each other. This is an electrostatic potential energy decrease. It would take mechanical work to pull them out of this well.
This is a blended classical/quantum description designed to help you conceptualize bond formation. Most chemical reactions involve the breaking of old bonds (internal energy increase) coinciding with formation of new bonds (internal energy decrease). Whether the system has lost or gained internal energy depends on whether the stronger bonds are the bonds broken or the bonds formed.
In forming a covalent bond, two atoms take a trip take down into a potential energy well. How deep is the well? That's the strength of the bond, i.e. how much energy the particle system loses when the bond is formed and how much energy would be required to break the bond.
The internal energy change of two atoms undergoing bond formation involves a decrease in electrostatic potential energy. The total energy of the bound molecule, with the atoms separated by the bond distance, is less than the energy of this simple system with the atoms fully separated. Why is this so?
Imagine bond formation. As electrons within nonbonded atoms form molecular orbitals, the nuclei are drawn inward along the bond axis and internal energy decreases. Try to picture it in your mind. As the bonding electrons form molecular orbitals, their presence within the internuclear space draws the nuclei inward. As the negatively charged electrons in the bonding orbital close distance with the positively charged nuclei on either side, unlike charges are drawing nearer to each other. This is an electrostatic potential energy decrease. It would take mechanical work to pull them out of this well.
This is a blended classical/quantum description designed to help you conceptualize bond formation. Most chemical reactions involve the breaking of old bonds (internal energy increase) coinciding with formation of new bonds (internal energy decrease). Whether the system has lost or gained internal energy depends on whether the stronger bonds are the bonds broken or the bonds formed.
In forming a covalent bond, two atoms take a trip take down into a potential energy well. How deep is the well? That's the strength of the bond, i.e. how much energy the particle system loses when the bond is formed and how much energy would be required to break the bond.