Techniques that make use the interactions of matter and electromagnetic radiation are critically important to the qualitative analysis of chemical substances. The most important techniques of molecular spectroscopy in the modern laboratory are infrared, NMR, ultraviolet, and mass spectroscopy. (With mass spectroscopy, the 'spectrum' refers to a distribution of ions sorted by weight.)
For the MCAT, firstly. you need to have a solid physical sciences understanding of the basis for how the techniques work. Secondly you need to have in hand the half dozen or so guideposts for basic interpretation of results within each method. Types of problems involving identification of an unknown on the MCAT are typically straightforward.
WikiPremed Resources
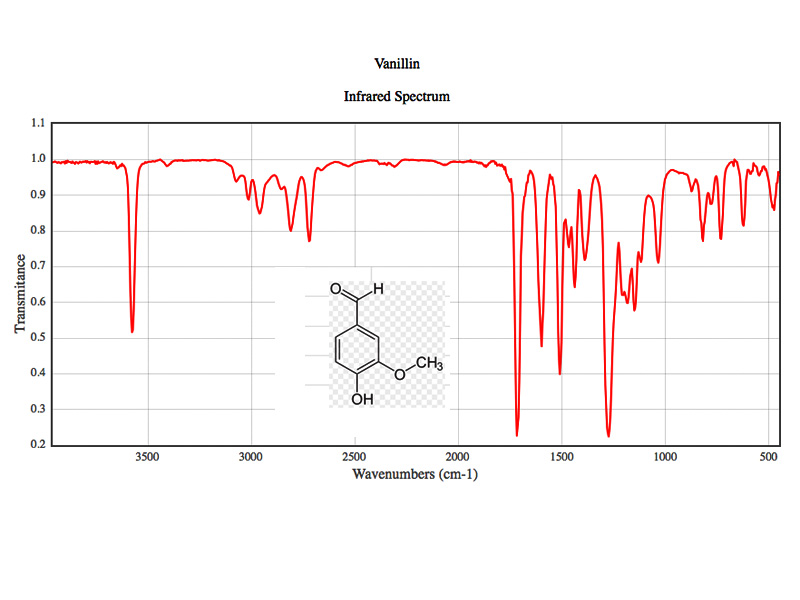
Molecular Spectroscopy Images
Conceptual Vocabulary Self-Test
Basic Terms Crossword Puzzle
Basic Puzzle Solution
Conceptual Vocabulary for Molecular Spectroscopy
Molecular Spectroscopy
Spectroscopy is the study of the interaction between radiation and matter.
A spectrometer is an optical instrument used to measure properties of light over a specific portion of the electromagnetic spectrum, typically used in spectroscopic analysis to identify materials.
Mass spectrometry is an analytical technique used to measure the mass-to-charge ratio of ions.
Infrared spectroscopy is a form of absorption spectroscopy that develops information about the structure of molecular substances from various covalent bond vibrational modes.
Nuclear magnetic resonance spectroscopy exploits the magnetic properties of certain nuclei. The most important applications for the organic chemist are proton NMR and carbon-13 NMR spectroscopy.
A material's absorption spectrum shows the fraction of incident electromagnetic radiation absorbed by the material over a range of frequencies.
Proton NMR is the application of nuclear magnetic resonance in NMR spectroscopy with respect to hydrogen.
A mass spectrum is an intensity versus mass-to-charge ratio plot which represents the distribution of components by mass-to-charge ratio in a sample.
J-coupling describes the interaction between two nuclear spins due to the influence of bonding electrons on the magnetic field running between the two nuclei.
In nuclear magnetic resonance (NMR), the chemical shift describes the dependence of nuclear magnetic energy levels on the electronic environment in a molecule.
Carbon-13 NMR is the application of nuclear magnetic resonance with respect to carbon.
The gyromagnetic ratio of a particle or system is the ratio of its magnetic dipole moment to its angular momentum.
Larmor precession refers to the precession of the magnetic moments of electrons, atomic nuclei, and atoms about an external magnetic field.
Tetramethylsilane is a commonly employed internal chemical standard for calibrating chemical shift in NMR spectroscopy.
Ultraviolet-visible spectroscopy uses electromagnetic radiation in the visible, UV, and near-infrared ranges to access electronic transitions in certain, usually conjugated, substances.
The Beer-Lambert law is an empirical relationship that relates the absorption of light to the properties of the material through which the light is traveling.
Alpha cleavage in the context of mass spectroscopy refers to the act of breaking the carbon-carbon bond, where one of the carbons bears a functional group.
An ion source is an electro-magnetic device that is used to create charged particles usually within mass spectrometers or particle accelerators.
Near infrared spectroscopy (NIRS) is a spectroscopic method utilising the region of the electromagnetic spectrum from about 800 nm to 2500 nm.
In nuclear magnetic resonance spectroscopy the term relaxation describes several processes by which nuclear magnetization prepared in a non-equilibrium state returns to the equilibrium distribution.
Molecular electronic transitions take place when valence electrons in a molecule are excited from one energy level to a higher energy level.
Bathochromic shift is a change of spectral band position in the absorption, reflectance, transmittance, or emission spectrum of a molecule to a longer wavelength.
Hypsochromic shift is a change of spectral band position in the absorption, reflectance, transmittance, or emission spectrum of a molecule to a shorter wavelength.
Rotational spectroscopy or microwave spectroscopy studies the absorption and emission electromagnetic radiation by molecules associated with a corresponding change in the rotational quantum number of the molecule.
The residual dipolar coupling between two spins in a molecule occurs if the molecules in solution exhibit a partial alignment leading to an incomplete averaging of spatially anisotropic dipolar couplings.
Electron ionization, formerly known as electron impact, is a technique widely used in mass spectrometry for generating ions, particularly for organic molecules.
Electrospray ionization (ESI) is a technique used in mass spectrometry to produce ions which is helpful in overcoming the propensity of macromolecules to fragment when ionized.
Tandem mass spectrometry involves multiple steps of mass spectrometry selection, with some form of fragmentation occurring in between the stages.
Relying on inelastic scattering of monochromatic light, Raman spectroscopy is a spectroscopic technique used in condensed matter physics and chemistry to study vibrational, rotational, and other low-frequency modes in a system.
Raman optical activity is a vibrational spectroscopic technique that is reliant on the difference in intensity of inelastically scattered right and left circularly polarised light due to molecular chirality.
Spin-lattice relaxation time is a time constant in NMR which characterizes the rate at which the longitudinal component of the magnetization vector recovers
Spin-spin relaxation time is a time constant in NMR which characterizes the rate at which the x-y component of the magnetization vector decays in the transverse magnetic plane.
The Rabi cycle is the cyclic behaviour of a two-state quantum system in the presence of an oscillatory driving field.
The McLafferty rearrangement is a phenomenon observed in mass spectrometry in which a molecule containing a keto-group undergoes beta-cleavage, with the gain of the gamma-hydrogen atom.
A sector instrument is a general term for a class of mass spectrometer that utilizes a static electric or magnetic sector or some combination of the two as a mass analyzer.
An important process for electrospray ionization in mass spectroscopy, a Taylor cone refers to the cone from which a jet of charged particles emanates above a threshold voltage.
Matrix-assisted laser desorption/ionization (MALDI) is a soft ionization technique used in mass spectrometry, allowing the analysis of biomolecules and large organic molecules which tend to fragment easily by other methods.
A quadrupole ion trap exists in both linear and 3D varieties and refers to an ion trap that uses DC and radio frequency oscillating AC electric fields to trap ions.
Electron capture dissociation is a method of fragmenting gas phase ions for tandem mass spectrometric analysis. ECD involves the direct introduction of low energy electrons to trapped gas phase ions.