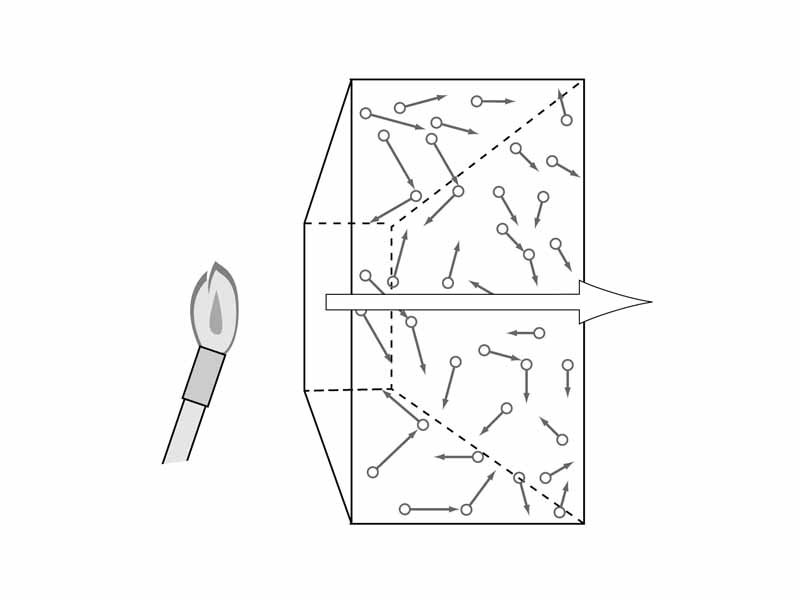
The concepts of Heat and Temperature are applied much more loosely in everyday life than in formal scientific discussion. In physics and chemistry, heat energy refers to thermal energy which is transfered from one object to another. In other words, heat energy is not the internal energy, or even the thermal energy of any object. Heat energy refers to the energy which is transfered in the process of heat flow. When you want to discuss the energy of particle motion within a substance itself, employ the concept of thermal energy, not heat. Thermal energy is the portion of an object's internal energy due to the object being at a temperature greater than absolute zero.
Temperature is also an everyday idea that has important conceptual substance in science. An object's temperature is an intrinsic property, but it can also be defined extrinsically, as predicting the direction of heat flow between objects. Reconciling the approaches to temperature within thermodynamics is a primary conceptual arc. For a single object, the temperature is a quantity that goes up or down with the average kinetic energy per particle. However, the particles of some substances have more places to put thermal energy (translation, vibration, rotation, etc) so the temperature is not a strict measure of kinetic energy per particle, but a measure of thermal energy per degree of freedom. The temperature difference between one object and another predicts the direction that heat will spontaneously flow. Heat flows spontaneously from a higher temperature object to a lower temperature object.
In addition to being important, fundamental material, Heat and Temperature is one of those topics where the basics do also appear in a straightforward way on the MCAT. While understanding this material is crucial to understanding what comes later in chemistry and biology, you also need to make sure to master fundamental problem solving in areas such as heat capacity, temperature scales, or heat transfer.
WikiPremed Resources
Heat & Temperature Cards
Conceptual Vocabulary Self-Test
Basic Terms Crossword Puzzle
Basic Puzzle Solution
Conceptual Vocabulary for Temperature & Heat Flow
Temperature & Heat Flow
Temperature is defined as the average energy of microscopic motions of a single particle in the system per degree of freedom.
Heat is energy transferred from one body or system to another due to a difference in temperature.
The kelvin is one of the seven SI base units. It corresponds to the absolute temperature scale where the coldest possible temperature is zero.
Heat conduction is the spontaneous transfer of thermal energy through matter.
A thermometer is a device that measures temperature or temperature gradient.
Zero on the Celsius scale was defined until 1954 as the melting point of ice and 100 degrees was defined as the boiling point of water under a pressure of one standard atmosphere. The definition is more formal today.
In the Fahrenheit scale, the melting point of water is 32 degrees and the boiling point is 212 degrees, placing the boiling and melting points of water exactly 180 degrees apart.
Absolute zero describes a theoretical system that neither emits nor absorbs energy whose temperature is zero Kelvin.
Heat transfer is the passage of thermal energy from a hot to a cold body.
One of the major modes of heat transfer, convection, refers in the most general terms to the movement of currents within fluids.
A calorie is a unit of measurement for energy. In most fields, it has been replaced by the joule. However, a thousand-fold variation remains in common use within the field of nutrition.
The Zeroth law of thermodynamics states that if two thermodynamic systems are in thermal equilibrium with a third, they are also in thermal equilibrium with each other.
Specific heat is the measure of the heat energy required to increase the temperature of a unit quantity of a substance by a certain temperature interval.
Thermal radiation refers to electromagnetic waves emitted from the surface of an object which is due to the object's temperature.
The emissivity of a material is the ratio of energy radiated by the material to energy radiated by a black body at the same temperature.
The Stefan-Boltzmann law states that the total energy radiated per unit surface area of a black body in unit time is directly proportional to the fourth power of the black body's thermodynamic temperature.
Thermal energy is the energy portion of a system that increases with its temperature.
Thermal conductivity, k, is the intensive property of a material that indicates its ability to conduct heat. It is used primarily in Fourier's Law for heat conduction.
Infrared radiation is electromagnetic radiation of a wavelength longer than that of visible light, but shorter than that of radio waves.
The Dulong-Petit law gives the classical expression for the specific heat capacity of a crystal due to its lattice vibrations.
R-value is a term used in the building industry to rate the insulative properties of construction materials. The higher the value, the greater the insulation.
Wien's displacement law states that there is an inverse relationship between the wavelength of the peak of the emission of a black body and its temperature.
Planck's law describes the spectral radiance of electromagnetic radiation at all wavelengths from a black body at a given temperature as a function of frequency.
Kirchhoff's law of thermal radiation states that at thermal equilibrium, the emissivity of a body (or surface) equals its absorptivity
In heat transfer analysis, thermal diffusivity is the ratio of thermal conductivity to volumetric heat capacity.
The Biot number is a dimensionless number used in unsteady-state heat transfer calculations to relate the heat transfer resistance inside and at the surface of a body.
Thermal contact conductance is the study of heat conduction between solid bodies in contact.
A heat pipe is a heat transfer mechanism that can transport large quantities of heat with a very small difference in temperature between the hotter and colder interfaces.
The heat capacity ratio or adiabatic index is the ratio of the heat capacity at constant pressure to heat capacity at constant volume.
The heat equation is a partial differential equation which describes the variation of temperature in a given region over time.
The Nusselt number is a dimensionless number that measures the enhancement of heat transfer from a surface that occurs in a 'real' situation compared to the heat transferred if just conduction occurred.