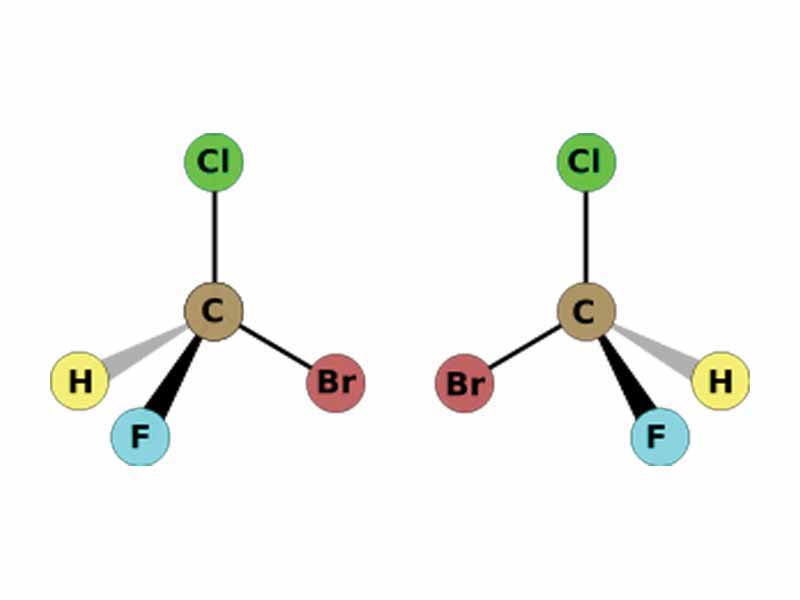
Stereochemistry is the branch of chemistry concerned with the spatial arrangements of atoms in molecules. Stereoisomers are molecules which do not differ in the sequential arrangement of atoms, but the spatial arrangment. In our earlier chapter, the Conformations of Organic Molecules, we were concerned with one sub-class of stereoisomerism, conformational isomerism, in which interconversion between the differing forms may occur without breaking bonds. In contrast, however, configurational isomers, the other type of stereoisomers, may not be interconverted without breaking bonds. If two configurational isomers are mirror images, they are enantiomers. Configurational isomers which are not mirror images are enantiomers.
Stereochemistry is a crucial topic for the MCAT. Nearly every exam will contain at least one question dealing directly with stereochemistry and several questions dealing indirectly. An MCAT passage presenting a reaction mechanism will likely be followed by at least one question dealing with issues such as inversion of configuration or racemization. Students are often required to recognize meso- forms on the MCAT or predict the number of stereoisomers a structure may have. Watch for stereochemistry to be presented on the MCAT in an unusual context such coordination chemistry.
WikiPremed Resources
Stereochemistry Images
Conceptual Vocabulary Self-Test
Basic Terms Crossword Puzzle
Basic Puzzle Solution
Conceptual Vocabulary for Organic Structure & Stereochemistry
Organic Structure & Stereochemistry
Stereochemistry involves the study of the relative spatial arrangement of atoms within molecules.
The term chiral is used to describe an object that is non-superimposable on its mirror image.
Cis-trans isomerism is a form of stereoisomerism describing the orientation of functional groups typically around double bonds which cannot rotate.
Steric effects arise from the fact that if atoms are brought too close together, there is an associated cost in energy due to overlapping electron clouds.
Enantiomers are stereoisomers that are nonsuperimposable complete mirror images of each other.
A racemic mixture is one that has equal amounts of left- and right-handed enantiomers of a chiral molecule.
Diastereomers are stereoisomers that are not enantiomers.
Optical rotation or optical activity is the rotation of linearly polarized light as it travels through certain materials.
A meso compound is a chemical compound with molecules that contain 2 or more stereocenters but which is optically achiral because it contains an internal plane of symmetry.
A Newman projection visualizes chemical conformations of a carbon-carbon chemical bond from front to back, with the front carbon represented by a dot and the back carbon as a circle.
A staggered conformation is a chemical conformation that exists in any open chain single chemical bond connecting two sp3 hybridised atoms as a conformational energy minimum.
An eclipsed conformation is a chemical conformation that exists in any open chain single chemical bond connecting two sp3 hybridised atoms as a conformational energy maximum.
A stereocenter is any atom in a molecule bearing groups such that an interchanging of any two groups leads to a stereoisomer.
Conformational isomerism is a form of stereoisomerism involving molecules with the same structural formula existing as different conformers due to atoms rotating about a bond.
Van der Waals strain results from van der Waals repulsion when two substituents in a molecule approach each other with a distance less than the sum of their van der Waals radii.
Cyclohexane is a cycloalkane containing 6 carbons and 12 hydrogens, which has the lowest angle and torsional strain of all the cycloalkanes.
The presence of angle strain in a molecule indicates that in a specific chemical conformation bond angles are deviating from the ideal bond angles required to achieve maximum bond strength.
Ring strain is an organic chemistry term that describes the destabilization of a cyclic molecule-such as a cycloalkane-due to the non-favorable high energy spatial orientations of its atoms.
Chiral resolution in stereochemistry is a process for the separation of racemic compounds into their enantiomers.
Prochiral molecules can be converted from achiral to chiral in a single step.
Hyperconjugation is the stabilizing interaction that results from the interaction of the electrons in a sigma bond with an adjacent empty or partially filled non-bonding p-orbital or antibonding pi orbital leading to an extended molecular orbital that increases the stability of the system.
The anomeric effect or Edward-Lemieux effect describes the tendency of heteroatomic substituents adjacent to a heteroatom within a cyclohexane ring to prefer the axial orientation instead of the expected, less-hindered equatorial orientation.
Enantiomeric excess exists where one enantiomer is present more than the other in a chemical substance.
Baeyer strain theory explains specific behaviour of chemical compounds in terms of bond angle strain.
Enantiomer self-disproportionation is a process describing the separation of a non-racemic mixture of enantiomers in an enantioenriched fraction and a more racemic fraction.
Homochirality is a term used to refer to a group of molecules that possess the same sense of chirality with similar groups are arranged in the same way around a central atom.
Chiral pool synthesis is a strategy that aims to improve the efficiency of chiral synthesis by beginning from a stock of readily available enantiopure substances.
Asymmetric induction describes the preferential formation in a chemical reaction of one enantiomer or diastereoisomer over the other as a result of the influence of a chiral feature present in the substrate, reagent, catalyst or environment.
A chiral auxiliary is a chemical compound or unit that is temporarily incorporated into an organic synthesis so that it can be carried out asymmetrically with the selective formation of one of two enantiomers.
Circular dichroism is a form of spectroscopy based on the differential absorption of left- and right-handed circularly polarized light.