In an alcohol, sp3 hybridized oxygen is bound to carbon. In sp3 hybridization, there are four regions of electron density. Picture the four regions of electron density around the oxygen atom within the hydroxyl group of an alcohol. The four regions of electron density surrounding oxygen contain the bonding electrons to carbon, the bonding electrons to hydrogen and two lone pairs.
Oxygen is the second most electronegative element on the periodic table (after fluorine) so the oxygen-carbon and oxygen-hydrogen bonds are both polar.
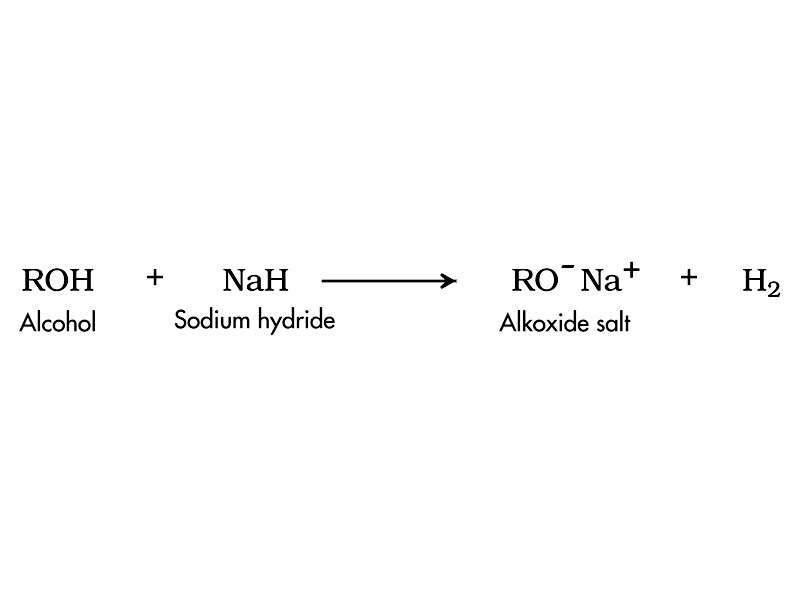
The oxygen-hydrogen bond is a source of reactivity through ionization to form alkoxide anion (either through acid-base equilibrium or reaction with metals).
The carbon-oxygen bond can be cleaved in substitution (reaction with HX or conversion to ethers) or elimination mechanisms (dehydration), or reaction with SOCl2 or PBr3.
A carbonyl group can be formed (oxidation of the alcohol) in reactions involving both the oxygen-hydrogen bond and a carbon-hydrogen bond on the adjacent carbon.
Oxygen is the second most electronegative element on the periodic table (after fluorine) so the oxygen-carbon and oxygen-hydrogen bonds are both polar.
The oxygen-hydrogen bond is a source of reactivity through ionization to form alkoxide anion (either through acid-base equilibrium or reaction with metals).
The carbon-oxygen bond can be cleaved in substitution (reaction with HX or conversion to ethers) or elimination mechanisms (dehydration), or reaction with SOCl2 or PBr3.
A carbonyl group can be formed (oxidation of the alcohol) in reactions involving both the oxygen-hydrogen bond and a carbon-hydrogen bond on the adjacent carbon.