A common genre of MCAT passage begins with discussion of the molecular biology or biochemistry context leading to the rationale for an enzyme assay. This section of the passage will be followed by an outline of procedures and results of the assay. Enzyme assays are laboratory methods for measuring enzymatic reaction rate or enzymatic activity. They are central to the study of enzyme kinetics and enzyme inhibition, for example. They can provide valuable insights into the structure of the substrate binding site or the enzymatic mechanism.
For example, if data from a table demonstrates that a point mutation has increased Michaelis constant, KM, it is reasonable to suppose that a sense mutation has occurred producing an amino acid substitution that has affected substrate binding. In the wild type enzyme, the original residue must be playing a role in substrate binding. Conversely, if the mutation has led to a decrease in Vmax, the mutation is affecting catalytic turnover. The mutation has led to a decrease in kcat. (Remember that kcat[ES] = Vmax when the enzyme is saturated.) If Vmax has decreased, it is reasonable to suppose that the residue played a role in catalytic turnover in the wild type enzyme. Identifying a residue essential for catalytic turnover may provide valuable insight into the enzymatic mechanism.
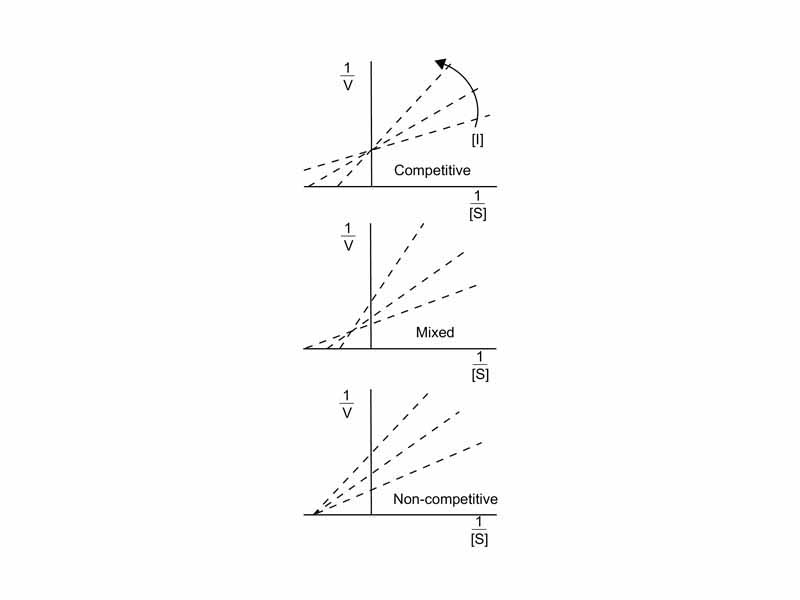
A similar logic applies for an assay in which the presence or absence of an inhibitor is the manipulated variable. If KM increases in the presence of the inhibitor while Vmax remains unchanged, you can suppose the inhibitor to be a competitive inhibitor, but if KM remained unchanged while Vmax decreased, it is likely the inhibitor is a noncompetitive inhibitor. However, let us point out that it is in the nature of the new MCAT for the data to show both KM and Vmax decreasing proportionally. That would indicate uncompetitive inhibition, a type of inhibition in which the inhibitor only binds the ES complex after the substrate is also bound. Finally, if KM increases while Vmax decreases, that would be consistent with mixed inhibition. Mixed inhibition is very similar to noncompetitive inhibition except that the bound inhibitor is not only affecting catalytic turnover but also has at least some effect on substrate binding.
Plots of catalytic rate versus pH might be used to determine which ionizable groups are participating in catalysis. When the rate undergoes a large alteration over a small pH range, it is a plausible though not a definitive supposition that those amino acid residues whose side chains with pKa's in that range are playing an important role.
By far the most common type of assay is what is known as an initial rate experiment. In other words, the researcher is measuring v0. In an initial rate experiment, reaction rate is measured right after adding substrate. It is so common for an assay to be done this way, that many educational resources will just write "v", for example, in the Michaelis-Menten equation, when they mean v0).
In the passage, data may be presented to you in the form of a table, Lineweaver-Burke plot, or a graph of initial rate versus substrate concentration. If the graph of rate versus substrate concentration is a hyperbola, that is consistent with Michaelis-Menten kinetics, but if the graph is sigmoidal, the enzyme-substrate binding is cooperative, and the enzyme is almost but not absolutely certain to be a multi-subunit allosteric enzyme. You can arrive at the same conclusion if a data table shows a Hill coefficient greater than 1.
Be careful to distinguish experiments to determine enzymatic activity from those simply determining reaction rate. In this context, enzyme activity has a specific meaning. Enzyme activity = moles of substrate converted per unit time = rate × reaction volume. You are measuring enzyme activity if you are trying to determine the amount of active enzyme present. This is not so common, but watch out because you are likely to be more accustomed to measurements that measure enzymatic reaction rate with a known amount of enzyme. One might measure enzyme activity, for example, to determine the purity of the enzyme within a protein mixture.
Enzyme assays measure either the consumption of substrate or production of product over time. There are many different ways to measure the concentrations of substrates and products, so pay careful attention to how measurement of the rate is operationalized in the experiment.
The assay might be continuous in which measurement follows the course of the reaction. A spectrophotometric assay follows the course of the reaction by measuring a change in how much light of a particular wavelength the assay solution absorbs. Alternatively, the assay might be fluorometric. A product or reagent might be fluorescent. The enzymatic reaction might actually be coupled with another reaction that produces a fluorescent product.
In contrast to a continuous assay, the assay might be discontinuous. In this type of procedure, the researcher is measuring the amount of reagent consumed or product formed at fixed intervals. Such an assay might include incorporation of a radionuclide in the reaction, such as 3H, 14C, 32P, or 35S. These are all β- emitters. They are are work-horses of the life sciences bench-top. Collected samples can be prepared for subsequent measurement in liquid scintillation counting, a very sensitive technique in which β- emissions from the sample produce countable bursts of light through interaction in the instrument within an lsc cocktail into which they have been emulsified.