Thermochemistry is the branch of chemistry concerned with the heat evolved or absorbed in a chemical process. Thermochemistry approaches chemical change through the First Law of Thermodynamics, but finds it more convenient to define a state function, the enthalpy, which is the sum of a system's internal energy and the product of its pressure and volume (H = E + PV).
The enthalpy is an extremely useful construct. We understand from the First Law of Thermodynamics that the heat flow occuring in a process such as mixing, phase change, or chemical reaction must be equal to the combination of internal energy change for the system and thermodynamic work. With the enthalpy change, though, we can describe the heat flow as a change in a single state function, the enthalpy. Because enthalpy change is not path dependent, but dependent on change in thermodynamic state, we can derive the extremely useful principle, Hess's Law of Heat Summation, which states that the heat exchange accompanying a transformation is the same whether the process occurs in one or several steps. A large part of skill in Thermochemistry involves imagining pathways for chemical change that help you understand the enthalpy change of a process which may or may not occur by that path.
The sequence of thermochemical reasoning is one of the primary conceptual arcs underlying the understanding of chemistry, so while there may only be a couple of questions that lead to an 'enthalpy change' as an answer, your thermochemical understanding will be in operation throughout the exam. When you learn to see the substances involved in a chemical reaction as a thermodynamic system, you can see any chemical process as a transformation through which internal energy may change, thermodynamic work may be performed, and heat may be evolved or absorbed. Over the next chapters, after we begin making the connections between Thermochemistry and Chemical Thermodynamics, knowledge of Thermochemistry will serve as the basis for a deeper understanding of spontaneity and chemical equilibrium.
WikiPremed Resources
Thermochemistry Practice Items
Conceptual Vocabulary Self-Test
Basic Terms Crossword Puzzle
Basic Puzzle Solution
Conceptual Vocabulary for Thermochemistry
Thermochemistry
Thermochemistry is the study of the heat evolved or absorbed in chemical reactions.
The enthalpy or heat content is a quotient or description of thermodynamic potential of a system equivalent to the sum of the internal energy of the system plus the product of its volume multiplied by the pressure exerted on it by its surroundings.
The heat of combustion is the energy released when a compound undergoes complete combustion with oxygen.
Exothermic describes a process or reaction that releases energy in the form of heat.
Endothermic describes a process or reaction that absorbs energy in the form of heat.
A calorimeter is a device used for measuring the heat of chemical reactions or physical changes as well as heat capacity.
Standard temperature and pressure is a standard set of conditions for experimental measurements, to enable comparisons to be made between sets of data.
Developed through conceptualizing cyclic reaction processes in which the return path is different than the forward path, Hess's Law of Heat Summation is used to predict the enthalpy change regardless of the path through which it is to be determined.
A reaction calorimeter is an instrument that enables the energy being released or absorbed by a reaction to be measured.
Calorimetry is the science of measuring the heat of chemical reactions or physical changes.
Bond dissociation energy is defined as the standard enthalpy change when a bond is cleaved by homolysis, with reactants and products of the homolysis reaction at 0K (absolute zero).
The standard enthalpy of combustion is the change in enthalpy of the total reacting system when one mole of a substance completely reacts with oxygen, and is observed at 298K and 1 atmospheric pressure
The standard enthalpy of formation of a compound is the change of enthalpy that accompanies forming 1 mole of a substance in its standard state from its constituent elements in their standard states
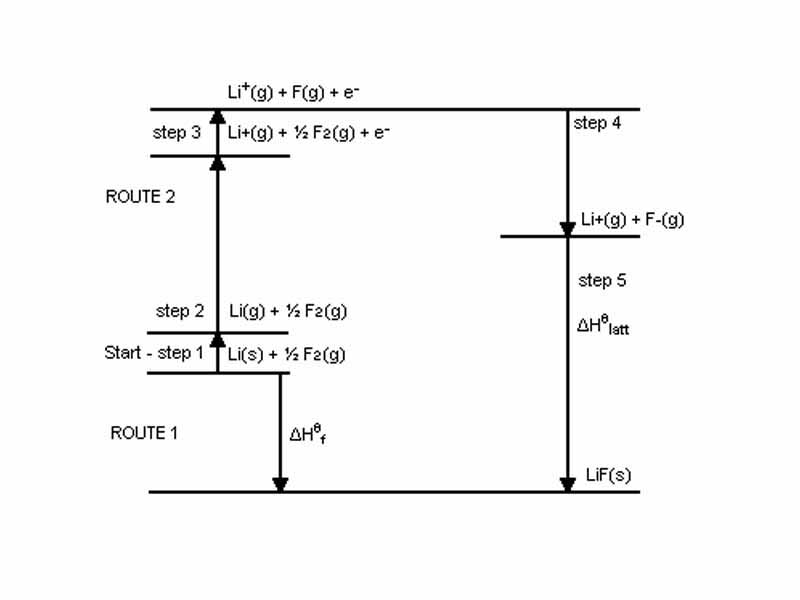
The Born-Haber Cycle is an approach to analyzing reaction energies involving the formation of an ionic compound from the reaction of a group I or group II metal with a non-metal.
The standard state of a material is its state at 1 bar (100 kilopascals exactly).
The lattice energy of an ionic solid is a measure of the strength of bonds in that ionic compound, equivalent to the amount of energy required to separate a solid ionic compound into gaseous ions.
The standard enthalpy change of reaction is the enthalpy change that occurs in a system when one mole of matter is transformed by a chemical reaction under standard conditions.
The enthalpy of atomization is the enthalpy change that accompanies the total separation of all atoms in a chemical substance
An isenthalpic process is one that proceeds without any change in enthalpy (also known as a throttling process).
An isodesmic reaction is a chemical reaction in which the type of chemical bonds broken in the reactant are the same as the type of bonds formed in the reaction product.