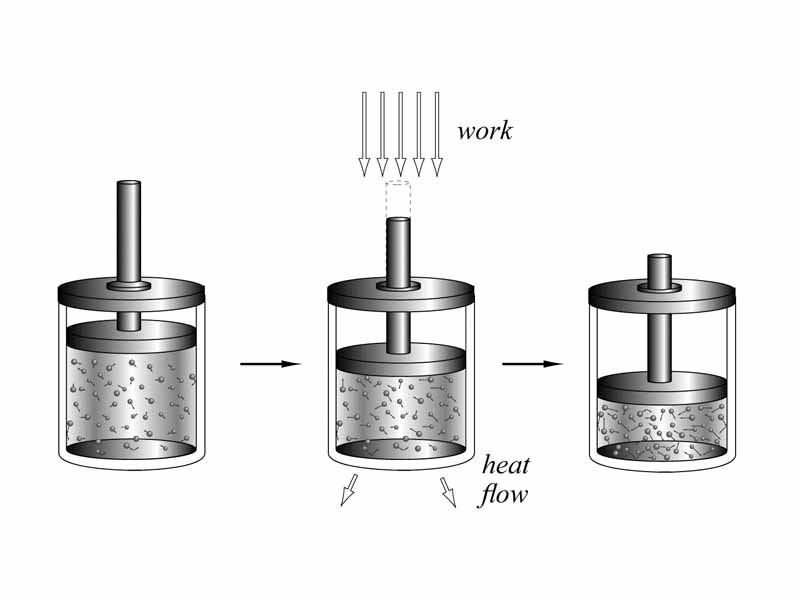
What does that mean for a physical system within its surroundings that the energy of the universe is constant? It means that as the system exchanges energy with its environment through heat flow or thermodynamic work (pressure-volume work), there must be a corresponding loss or gain to the internal energy of the system. Think about it. This is just common sense. If heat is flowing into a system at constant volume (no work), the internal energy of the system must be increasing. If heat is flowing out, the internal energy of the system must be decreasing. If the surroundings perform work on the system and heat flow is prevented, the change in internal energy must equal the work that is being done on the system. Usually there is some combination of heat flow and work occuring in a thermodynamic transformation. Sum it up (keep the plus and minus signs straight) and the internal energy change will equal the total. The first law of thermodynamics is the easy part of thermodynamics.
Whether an MCAT passage deals with a steam engine or a phase diagram, if an MCAT passage has a theme directly concerned with thermodynamics, there will be a question or two requiring you to reason from the 1st Law. That is important enough, but it is trivial compared to the significance of understanding thermodynamics for understanding science. Stick with it here. Really concentrate. When we move through the chemistry and into biochemistry later in this course, there will be a big payoff in your conceptual understanding if you really push at every stage in the thermodynamics now to understand in a concrete, intuitive way.
WikiPremed Resources
1st Law of Thermodynamics Cards
Conceptual Vocabulary Self-Test
Basic Terms Crossword Puzzle
Basic Puzzle Solution
Conceptual Vocabulary for 1st Law of Thermodynamics
1st Law of Thermodynamics
Thermodynamics is a branch of physics that studies the effects of changes in temperature, pressure, and volume on physical systems at the macroscopic scale by analyzing the collective motion of their particles.
The first law of thermodynamics states that the increase in the internal energy of a thermodynamic system is equal to the amount of heat energy added to the system minus the work done by the system on the surroundings.
The conservation of energy states that the total amount of energy in any system remains constant, although it may change forms.
Mechanical work is the amount of energy transferred by a force.
An isothermal process is a thermodynamic process in which the temperature of the system stays constant.
Heat transfer is the passage of thermal energy from a hot to a cold body.
The internal energy of a thermodynamic system is the total of the kinetic energy due to the motion of molecules and the potential energy associated with the vibrational and electric energy of atoms within molecules or crystals.
An isolated system, as contrasted with a open system, is a physical system that does not interact with its surroundings.
An isobaric process is a thermodynamic process in which the pressure stays constant.
An isochoric process, also called an isometric process or an isovolumetric process, is a thermodynamic process that occurs without a change in volume.
An adiabatic process or an isocaloric process is a thermodynamic process in which no heat is transferred to or from the working fluid.
A thermodynamic system, originally called a working substance, is defined as that part of the universe that is under consideration, separated by a real or imaginary boundary from the environment or surroundings
A thermodynamic state is the macroscopic condition of a thermodynamic system as described by its particular thermodynamic parameters.
A thermodynamic process may be defined as the evolution of a thermodynamic system proceeding from an initial state to a final state.
The mechanical equivalent of heat was an expression of 19th century science stating that mechanical work may be transformed into heat, and conversely heat into work, with the magnitude of one always proportional to the other.
In thermodynamics, work is the quantity of energy transferred from one system to another without an accompanying transfer of entropy.
A state function is a property of a system that depends only on the current state of the system, not on the way in which the system got to that state.
A open system describes a system in continuous interaction with its environment.
A closed system can exchange heat and work with its surroundings but not matter. This is in contrast to an isolated system which can exchange neither heat nor matter with the surroundings.
Phenomenological thermodynamics is a branch of thermodynamics concerned with the study and analysis of actual phenomena without evaluation of statistical energy-level atomic and molecular details.
A polytropic process is a thermodynamic process in which the logarithm of the pressure versus the logarithm of the volume is a straight line.