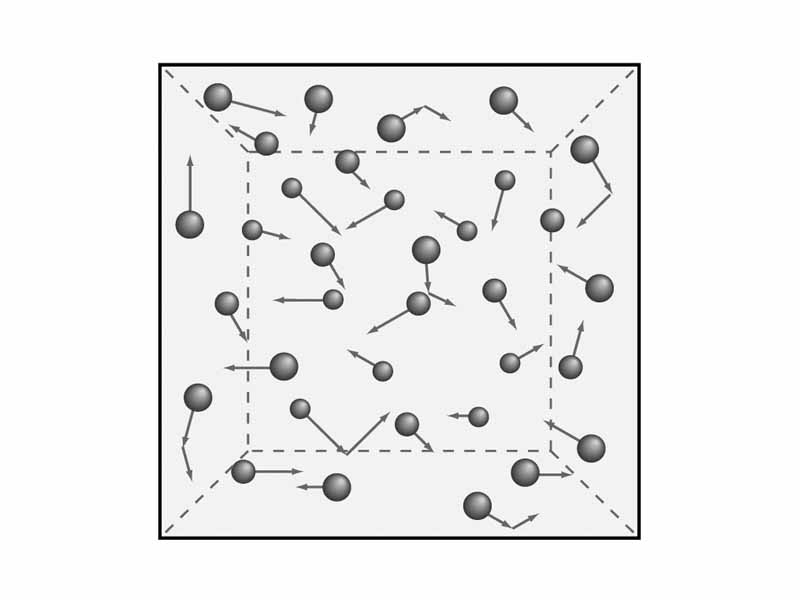
The thermodynamic state functions, pressure and temperature, are statistical quantities. They describe the 'macrostate' of a system of a very large number of particles. If you imagine a tiny plane situated at a point within a gas or liquid, the force exerted per unit area on that plane is the pressure at that location in the sample. (The 'tiny plane' terminology is to define pressure as a formal, intrinsic property of the fluid). Pressure is produced on a surface as the particles of the gas collide and rebound off the area. Think about pressure in terms of basic mechanics. The force generated at the microscopic level corresponds to the change in momentum of the rebounding particles. Think about it. A particle collides with the wall of the container and rebounds. Its momentum has changed. This means that force must have been exerted on the particle by the wall of the container, an impulse. The pressure felt by the wall is the reaction force to one it exerts on the particle. Now think about what would cause the pressure within a fluid (gas or liquid) to be greater. Pressure increases with fluid density (there are more particles colliding on a given area) and pressure increases with temperature (if the particles are moving faster, the microscopic changes in momentum associated with collisions will be greater).