In the earlier chapters of Atomic Theory, Periodic Properties, Chemical Bonding, and Intermolecular Forces, we discussed the structure of matter and some of the changes in structure that can occur at the atomic, molecular, or intermolecular level. It is important to remember, however, that Chemistry is a laboratory practice, and the scale of the laboratory is much larger than atoms and molecules. In order to describe the measurable relationships governing chemical change, a system of accounting is necessary which describes the products and reagents in chemical equations in measurable terms like mass and moles. Stoichiometry describes the body of accounting techniques for the purpose of describing chemical reactions at the laboratory scale.
It is the right stage of the course to take the time to cover Stoichiometry. Already this week in the Main Sequence, we have learned how to describe the most basic kind of physical thermodynamic system, an Ideal Gas within its surroundiongs, and now we need to equip ourselves with Stoichiometry to begin building the bridge to systems composed of real substances and the chemical reactions such systems may undergo.
Stoichiometric problem solving is a major focus during the first semester of General Chemistry, and many students get the impression of chemistry as an endless variation on plugging and chugging grams and moles, and their conceptual understanding of chemistry can suffer. Although this type of problem solving is relatively minor on the MCAT (MCAT physical science questions are much more likely to involve conceptual reasoning than number crunching), there will be enough stoichiometric terminology to prevent from achieving a superior score if they don't understand it. You definitely need to understand stoichiometric nomenclature and the basics of stoichiometric problem solving for the MCAT.
WikiPremed Resources
Stoichiometry Practice Items
Conceptual Vocabulary Self-Test
Basic Terms Crossword Puzzle
Basic Puzzle Solution
Conceptual Vocabulary for Stoichiometry
Stoichiometry
Stoichiometry is the calculation of quantitative relationships of the reactants and products in chemical reactions.
The mole is the SI base unit that measures an amount of substance, equal to Avogadro's number of entities.
Although the definition is more formal now, the gram was originally defined as the absolute weight of a volume of pure water equal to the cube of the hundredth part of a metre, and at the temperature of melting ice.
A reactant or reagent is a substance consumed during a chemical reaction.
A product is a substance that forms as a result of a chemical reaction.
A chemical reaction is a process that results in the interconversion of chemical substances.
Avogadro's number is the number of entities in one mole
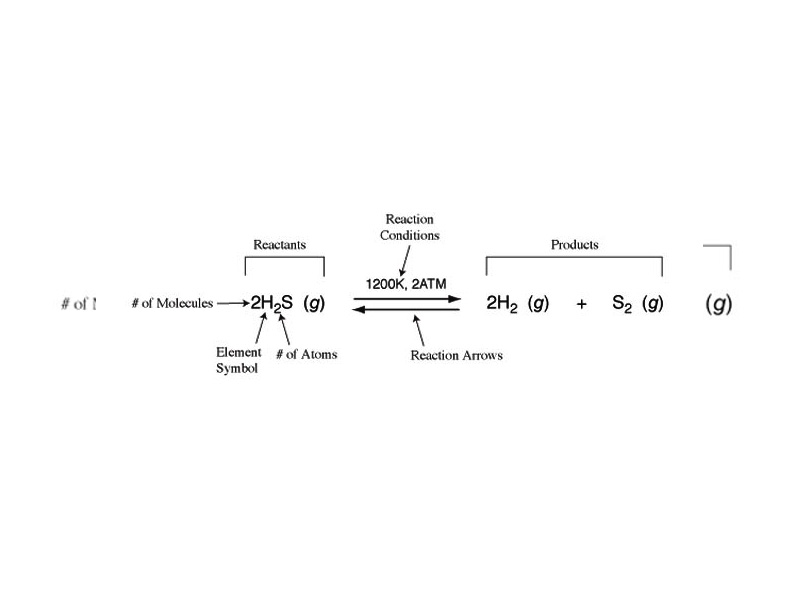
A chemical equation is a symbolic representation of a chemical reaction.
The law of conservation of mass states that the total amount of matter within a closed system will remain constant, regardless of the processes acting inside the system.
The molecular mass of a substance is the mass of one molecule of that substance, relative to the unified atomic mass unit (equal to 1/12 the mass of one atom of carbon-12).
The atomic mass is the mass of an atom at rest, most often expressed in unifed amu.
Yield is the amount of product obtained in a chemical reaction.
The limiting reagent is the chemical that determines how far a reaction would go because the chemical in question is the reagent that would get completely used up, causing the reaction to stop.
Gram atomic mass is the mass in grams of one mole of atoms in an element.
A chemical formula is a concise way of expressing information about the atoms that constitute a particular chemical compound.
The structural formula of a chemical compound is a graphical representation of the molecular structure showing how the atoms are arranged.
The law of definite proportions states that a chemical compound always contains exactly the same proportion of elements by mass.
The empirical formula of a chemical compound is a simple expression of the relative number of each type of atom in it.
The equivalent is a measurement unit used in chemistry and the biological sciences, which measures of a substance's ability to combine with other substances, an expression frequently used in the context of normality.
Atom economy describes the conversion efficiency of a chemical process in terms of all atoms involved.
A gram equivalent is the mass in grams of a compound's equivalent weight.
Lorenzo Avogadro (1776 - 1856) was an Italian scientist most noted for his contributions to the theory of molarity and molecular weight.
The law of multiple proportions, sometimes called Dalton's Law, states that if two elements form more than one compound between them, then the ratios of the masses of the second element which combine with a fixed mass of the first element will be ratios of small whole numbers.
Non-stoichiometric compounds are chemical compounds with an elemental composition that cannot be represented by a ratio of well-defined natural numbers, and therefore violate of the law of definite proportions.
Equivalent weight is the amount of an element that reacts with 1 mole of electrons.
A mass balance is an application of conservation of mass to the analysis of physical systems.