Interdisciplinary Note (9 of 16)
Oxidation-reduction is a set of accounting methods that provide a systematic approach to easily keep track of the shifts through that occur through a reaction. A chemical system is an electrostatic system. Oxidation-reduction keeps track of the valence electrons before and after the reaction. In this system, oxidation is defined as a process by which oxidation number increases, corresponding to a loss of electron control on the part of an element. In reduction oxidation number decreases, corresponding to a gain of electron control.
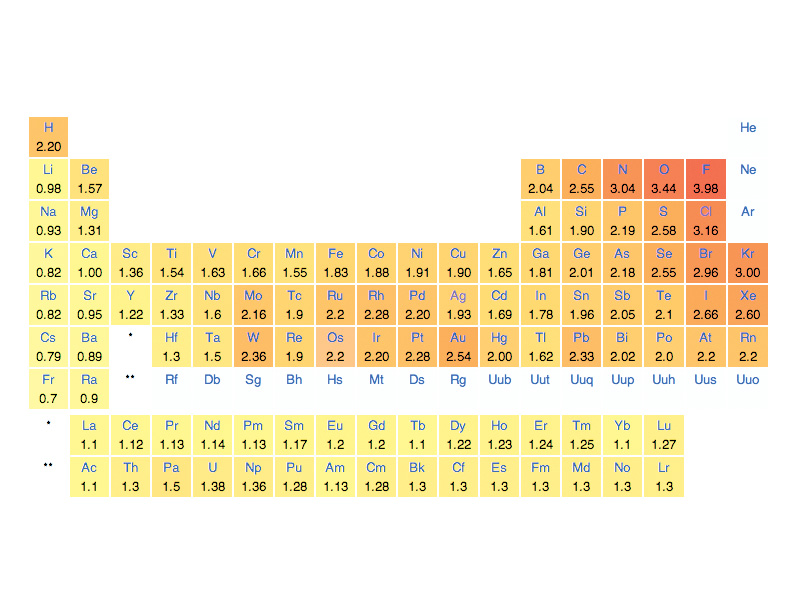
In order to assign electron control across covalent bonds, it is essential to know which atoms are more electronegative in the bonds. How electronegativity differences play out in chemical reactions provides the underlying coherence of the oxidation-reduction system. Electronegativity reflects the strength of attraction an atom has for the electrons it shares in chemical bonds, while oxidation-reduction is a systematic accounting procedure to reflect the changes in the bonding environment of electrons between products and reagents.
When two atoms form a covalent bond, the more electronegative atom is assigned 'electron control' in the oxidation-reduction system. If an atom gains electron control through a chemical process, it is said to be 'reduced,' while the atom that has lost electron control is said to be 'oxidized'. The key to the system is that when a very electronegative atom is reduced, it draws the new electrons inwards towards its strongly attracting nucleus, and the bond becomes polarized. This closing of separation between unlike charges represents a potential energy decrease above and beyond the typical energy decrease that accompanies the formation of an ordinary bonding orbital. In other words, the formation of polar bonds corresponds to large potential energy decreases (tending toward negative internal energy change, negative enthalpy, negative free energy change), and as a general rule, polar bonds are stronger than nonpolar bonds (more energy is required to break them because the electrons have to be wrenched away from the oxidant). Oxidation-reduction provides a systematic way to account for these tendencies. The more electronegative elements form stronger covalent bonds. We keep track of the number of electrons that have been drawn in towards its nucleus with the shifts in oxidation state, and we understand the energy involved in the transition per unit charge through the difference in reduction potential in volts between the destination of the electrons, the product or oxidant, and their origin, the reagent or reductant.