Interdisciplinary Note (8 of 16)
In performing practice items and passages, work on making it natural to recognize whether or not the oxidation numbers are changing between reagents and products in any reaction you are presented with. You want to recognize this right away, whether it is a redox reaction. It should be an integral part of "clearing" the reaction mentally while you are reading the passage. If oxidation numbers are changing, you know it's an oxidation-reduction reaction. You say, okay, hydrogen ions are oxidizing the aluminum ions, plating out aluminum metal and yielding hydrogen gas. Okay, NAD+ is oxidizing malate, becoming NADH. The hydroxyl group in malate became the carbonyl in oxaloacetate. It takes practice, but this really pays off. It makes what's going on in the passage intelligible and you are often already answering a question that's coming.
There are two main ways to assign oxidation numbers. Which one to use usually depends on whether you are presented with a molecular formula or a structural formula. Molecular formulas are what you get in the stoichiometry of a chemical equation involving inorganic reagents. On the other hand, you usually get structural formulas, pictures actually showing the bonds, in the organic chemistry and biochemistry contexts.
With molecular formulas and inorganic reagents, the general practice is to solve the oxidation numbers by working in on things with your rules. The rules give you enough a foothold in what you know to determine something which might be variable, such as the oxidation number of transition metals which have multiple common oxidation states. Assign the elements in the formula you can be sure of first and then lastly apply the rule that the sum of oxidation numbers has to equal zero in a neutral species, or equal the ion charge in a polyatomic ion. For example, with KMnO4, the common oxidizing agent, we could start with K. We know that K has an oxidation of number +1 (alkali metals are always +1 in compounds). We also know that O will be -2 (oxygen is almost always -2 in compounds). From just that, we know then the Mn must have an oxidation state of +7, ie. (+1) + (+7) + (4 × -2) = 0.
With structural formulas, the process is different. You look at each bond and decide who has "control" over the electrons shared in the bond. In a way, this is a "better" way to do it in a conceptual sense. Although they are much faster with a simple inorganic compound, the rules have a way of detaching redox from its conceptual underpinnings. Nevertheless, it's impossible to use the rules with the molecular formula of many organic compounds, because the carbons, at least, will often be at different oxidation states in different parts of the molecule. With structural formulas, you decide which of the two elements has "control" of electrons shared in the bond between them. The decision is based on electronegativity. The higher electronegativity element, even if it's only higher by a little bit, is assigned electron control.
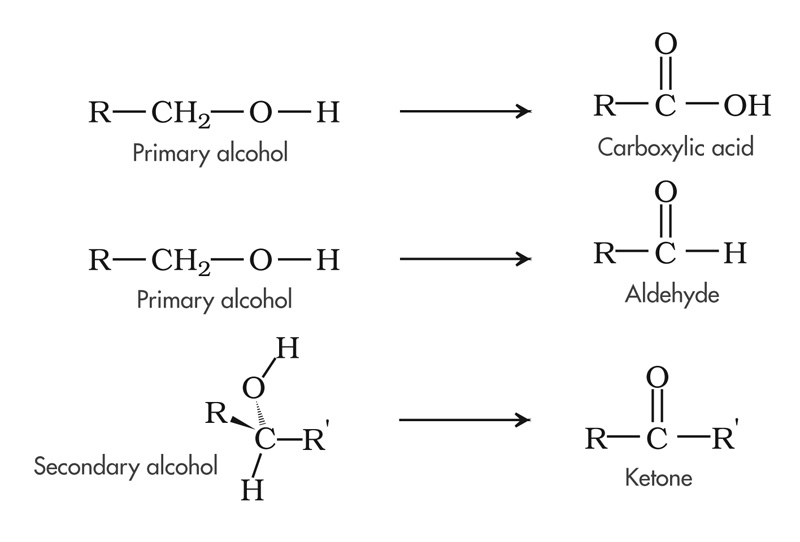
A good exercise is to look at the oxidation of a primary alcohol to form an aldehyde. The terminal carbon in a primary alcohol has two bonds to hydrogen, one to carbon, and one to another carbon. In forming the bonds to hydrogen, carbon gained the electrons each hydrogen brought, so that's -2. You know this because carbon's electronegativity is 2.5. Hydrogen's is 2.1. Carbon wins. On the other hand, carbon lost the electron it brought to its single bond to oxygen, whose electronegativity is 3.5, so that's +1 for the carbon in that bond. Lastly, there's no gain or loss in its bond to carbon. The net gain and loss (-2 + 1) is -1. That is the oxidation state of the carbon in a primary alcohol.
In an aldehyde, the carbon is in a double bond to oxygen, and it has only one bond to hydrogen, so it lost 2 electrons to oxygen and gained 1 from hydrogen, so it's oxidation state is +1.
If you oxidized the aldehyde further, or had begun with a more powerful oxidizing agent on the bench-top, you would form a carboxylic acid, and the carbon would now have an oxidation state of +3.