Interdisciplinary Note (10 of 16)
You can narrate the overall chemical changes of oxidative metabolism through the lens of chemical thermodynamics or you can narrate them through the lens of oxidation-reduction. From the thermochemical perspective, oxidative metabolism involves breaking relatively weak carbon-hydrogen and carbon-carbon bonds and forming stronger carbon-oxygen and hydrogen-oxygen bonds, bonds with greater electronegativity difference than the initial carbon-hydrogen and carbon-carbon bonds of carbohydrate or lipid. In forming the new polar, covalent bonds of the product, the powerful oxygen nucleus draws the valence electrons brought by carbon and hydrogen in towards itself. This is an internal energy decrease (which translates to an enthalpy decrease and a free energy decrease, energy ultimately translating to food calories). Because triglycerides have primarily carbon-hydrogen bonds, these molecules have a greater reservoir of electrons which can ultimately participate in bonds with oxygen. From a thermochemistry perspective, we are breaking weak bonds and forming strong bonds. From an oxidation-reduction perspective, oxygen is being reduced. When you can see that this is two ways of saying the same thing, you are seeing the underpinnings of redox.
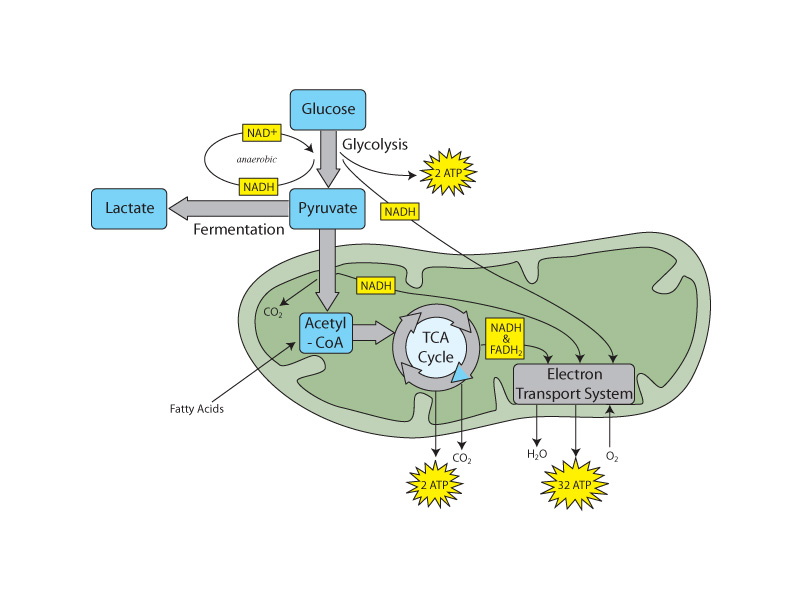
The successive changes that occur upon the glucose substrate through the various stages of oxidative metabolism (glycolysis, mobilization of pyruvate, the citric acid cycle) involve the oxidation-reduction series comprising alcohol, aldehydes & ketones, carboxylic acids, and carbon dioxide. Electrons in oxidative phosphorylation also make their way to oxygen in water. Oxidative metabolism can be envisioned as a narrative of electron control in which electronegative oxygen is gaining control of bonding electrons that were originally under the control of carbon. When oxygen forms a bond with a less electronegative element (any except fluorine) the electrons are drawn in toward the powerful oxygen nucleus, leading to the polarity of the bond and corresponding to internal energy decrease beyond ordinary bond energy. The fact that polar bonds are exceptionally strong (requiring the input of large amounts of energy to break), is the reason that energy is liberated in the oxidative metabolism of nutrient molecules. As the system moves from relatively weak C-H and O-O bonds to strong C-O and H-O bonds, the internal energy decrease translates to the free energy decrease that is coupled ultimately with the phosphorylation of ADP to form ATP.