Interdisciplinary Note (7 of 16)
The oxidation number system is like long division. Long division is a 'method' that gets you to the correct answer, but it can become completely detached from the conceptual underpinnings. You move the decimal point here. You keep your columns straight. You follow the steps, and don't have to really think about what is going on underneath. You get the answer.
Oxidation-reduction is analogous to long division within chemistry. You get to the answer without seeing the underpinnings of the system. This can turn chemistry into a play of floating signifiers that don't point to anything. Defamiliarize your perspective. Look at it in the plainest terms possible. On one side of the reaction you have charge densities, nuclei and electrons, That's one form things can take, the reagents. On the other side of the reaction, these charge densities are in a different form, the products.
As we have discussed, you can imagine the electrostatic potential energy change from reagents to products by imagining pulling apart the reagents and letting them fall together into the products. If the volume and temperature are constant, this electrostatic potential energy change maps directly onto enthalpy change.
If the chemical bonds in the reagents are weak, it does not take a great deal of energy to break them apart, and if the chemical bonds in the products are strong, a great deal of energy is released as the nuclei and electrons fall together into the deep potential energy wells of the new bonds. Such a reaction would have large, overall negative enthalpy change. What does this have to do with oxidation-reduction?
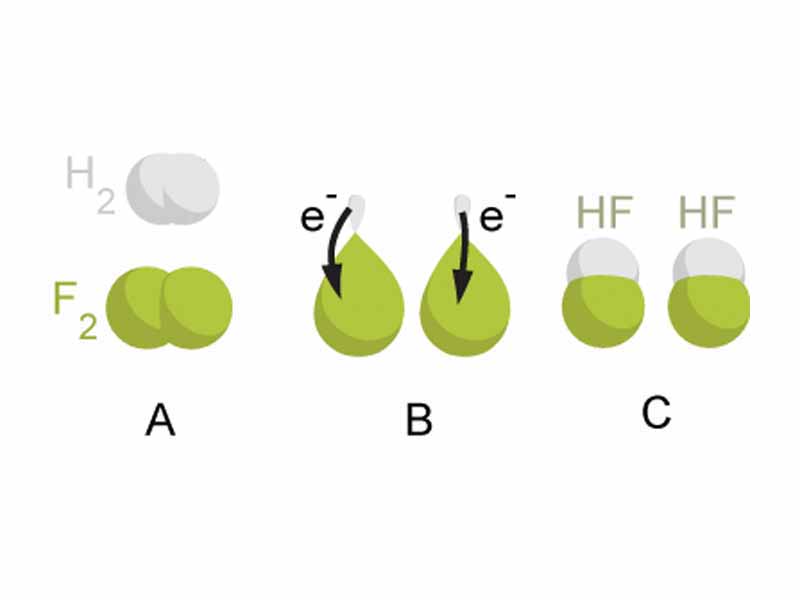
Oxidation-reduction is a system that chemists have developed, which allows you to easily predict the same analytical result in a simple step-like way. When an element with a high reduction potential (high electronegativity) forms a new covalent bond, it pulls the electron density within the bonding orbital inwards towards its powerful nucleus. Elements like oxygen or fluorine have a big powerful nucleus shielded by only a thin layer of electrons, so when they form new bonds, there is a potential energy decrease not only because of molecular orbital formation, but also because they are 'siezing electron control', i.e. pulling negative charge inwards. You would have to do a lot of work to get the electrons away from such an element, so very electronegative, high reduction potential elements form strong, low energy bonds.
You keep track of these kinds of shifts in 'electron control' using oxidation numbers. Oxidation-reduction gives one an easy way to account for the energy changes that occur through a chemical reaction as a narrative of electron control. The oxidation state or oxidation number of an element is a shorthand way of accounting for the shifts (or transfers) of electron density from one atom to another when compounds form. When the electron density is shifted toward the more electronegative element, that atom is said to have gained the electrons in redox terms, and its oxidation number decreases; the element having lost electron control in the compound gets a positive change to oxidation number. You can then apply electrochmical methods to determine the change in free energy as a fall of charged particles through a potential difference.