Interdisciplinary Note (22 of 29)
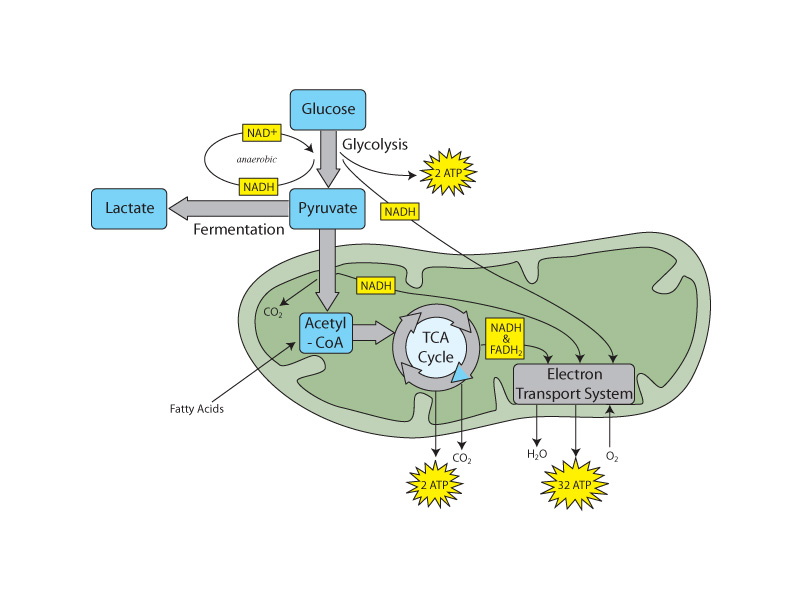
The successive changes that occur upon the glucose substrate through the various stages of oxidative metabolism (glycolysis, pyruvate dehydrogenase, the citric acid cycle) move through the oxidation-reduction series comprising alcohol, aldehydes & ketones, carboxylic acids, and carbon dioxide. Oxidative metabolism can be seen as a narrative of electron control in which electronegative oxygen is gaining control of bonding electrons that had been earlier under the control of carbon in glucose.
When oxygen forms a bond with a less electronegative element (any on the periodic table except fluorine) the electrons are drawn in towards the powerful oxygen nucleus. Everybody learns that the electronegativity of oxygen leads to polar bonds, but this internal shift, taken by itself, corresponds to an extra internal energy decrease beyond run-of-the-mill bond energy. The electrons are falling into a deeper potential energy well, drawn in towards the powerful nucleus of oxygen. Extra energy is lost when electronegative elements form bonds. The fact that polar bonds are exceptionally strong (requiring the input of large amounts of energy to break), is the reason that energy is liberated when they are formed. This is the energy driving the oxidative metabolism of nutrient molecules. As the system moves from C-H and O-O bonds to strong C=O and O-H bonds, internal energy decrease occurs, which translates to a free energy decrease. Oxidative metabolism harnesses the free energy decrease, coupling the process with ATP synthesis through both direct substrate level phosphorylation and oxidative phosphorylation in the mitochondria.
Let's use a gravitational analogy. Picture a water wheel being used to power a simple crane which is stacking bricks. The water wheel couples the decrease in potential energy of water with the increase in potential energy of the bricks. In metabolism, electrons are 'falling' towards the oxygen nucleus as new bonds are formed. The 'fall of electrons' is analogous to water falling along the incline with gravity. The water wheel couples the decrease in potential energy of water with the increase in potential energy of the bricks. The processes of metabolism couple the decrease in potential energy of electrons in metabolism with an increase of chemical energy elsewhere, in ATP formation, as the negatively charged phosphate group is pushed onto negatively charged ADP, a potential energy increase like compressing an electrostatic spring. Later, when the ATP formed this way comes apart under enzyme catalysis, the process will be coupled with one of a multitude of biological processes which would otherwise be nonspontaneous.