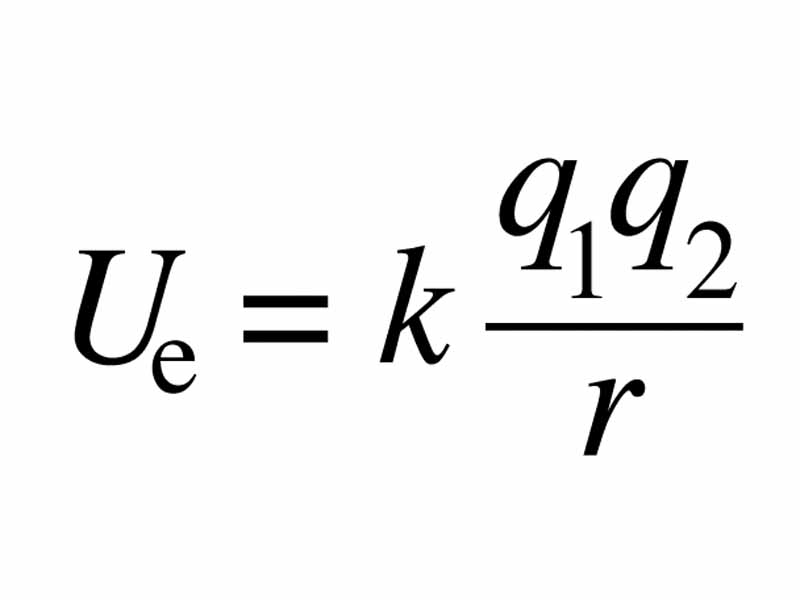Interdisciplinary Note (1 of 29)
An important idea from the first semester of organic chemistry is that the more substituted a carbocation the more stable it will be (tertiary > secondary > primary) because alkyl groups "donate" negative charge by induction along the bond axes towards the cation, stabilizing it. The more alkyl substituents present, the more negative charge density can be drawn in towards the positive charge
Our thermochemical language can help us give the idea in a bit more depth. Remember from electristatics that the closing of separation between unlike charges represents a decrease in electrostatic potential energy. The electrostatic potential energy decrease at the particle level translates to lower internal energy at the system level. Lower internal energy translates to lower enthalpy, and, ultimately, a lower free eenrgy. The free energy differences between a tertiary and a secondary carbocation is about 50 kJ mol-1.