Interdisciplinary Note (21 of 29)
One of the most salient characteristics of the hydroxyl group is that it is a very poor leaving group. Why is hydroxyl group a poor leaving group? The easy answer is that this is because hydroxide is a strong base. As a counter example, the very weak base bromide is a good leaving group. It's such a weak base you don't even think of it as a base, and it's a great leaving group. Weak bases make good leaving groups. Strong bases make bad leaving groups.
While this is a good rule of thumb, it doen't really answer the original question about why. Hydroxyl group's is a poor leaving group for the same reason it's a strong base. These are two sides of the same coin. Hydroxide is like angry water. Think about it thermodynamically. There is the state before leaving, and there is the state after. There is a free energy change. Hydroxide doesn't leave spontaneously because the second state at the system level is too high free energy. This is because it is too high in enthalpy, which is because it is too high in internal energy. The high internal energy at the system level is because hydroxide ion is too high in electrostatic potential energy at the particle level. Why is that? Why is hydroxide so high energy? Think about two metal spheres with the same amount of negative charges spread out evenly on their surfaces. The large sphere is bromide. The small sphere is hydroxide. The same electric charge can spread out on the large sphere at lower potential energy. Hydroxide is high energy because it's too small for the negative charge to spread out. The second shell octet is too crowded. This is why hydroxide is a strong base and also why it is a poor leaving group.
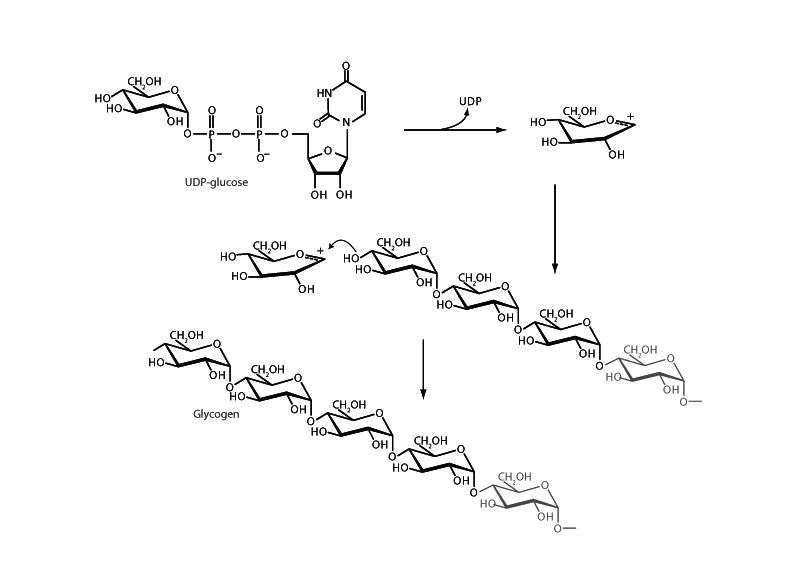
That hydroxide is a poor leaving group is a major concern for both organic chemistry and biochemistry. To get hydroxyl group to leave, you can employ acid catalysis on the benchtop, so that hydroxyl group can leave as water, or you could turn the alcohol into a sulfonyl ester where hydroxyl can leave as a mesylate or tosylate. In biochemistry, there is a similar logic to activation of a sugar for glycosidic bond formation with UDP.