Interdisciplinary Note (23 of 29)
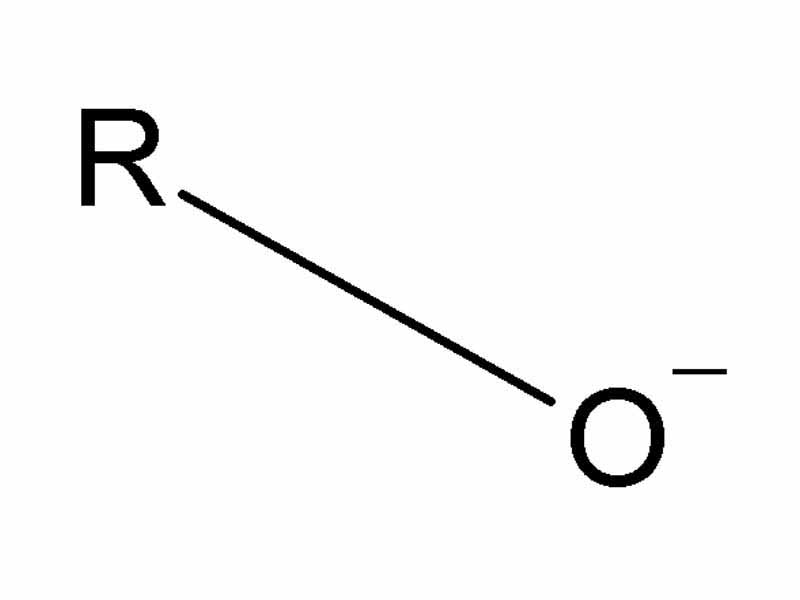
Alcohols are quite weak acids, and the conjugate base of an alcohol, alkoxide anions, are strong bases. Alkoxide is a stronger base than than hydroxyl. Ka = 10-16 for a primary alcohol and 10-18 for a tertiary alcohol. Because the Ka of typical alcohols is somewhat low for alkoxide anions to be easily formed by reaction with a strong base, alkoxide bases are usually formed by reaction of the alcohol with a group I metal (sodium or potassium). Alkoxide anions may be employed as nucleophiles in reactions such as nucleophilic substitution (to form ethers, i.e. Williamson Ether Synthesis), epoxide ring opening, or hemiacetal formation (under basic conditions not proceeding to complete acetal formation).
Some reactions, such as hydrolysis of an ester, may involve alkoxide as a leaving group. For the same reason that hydroxide is a poor leaving group, alkoxide is high energy and a poor leaving group too. In biochemistry, acid catalysis by a proton donating active site residue, histidine in chymotrypsin or glutamic acid in lysozyme, facilitates alkoxide to leave as alcohol.