Interdisciplinary Note (26 of 29)
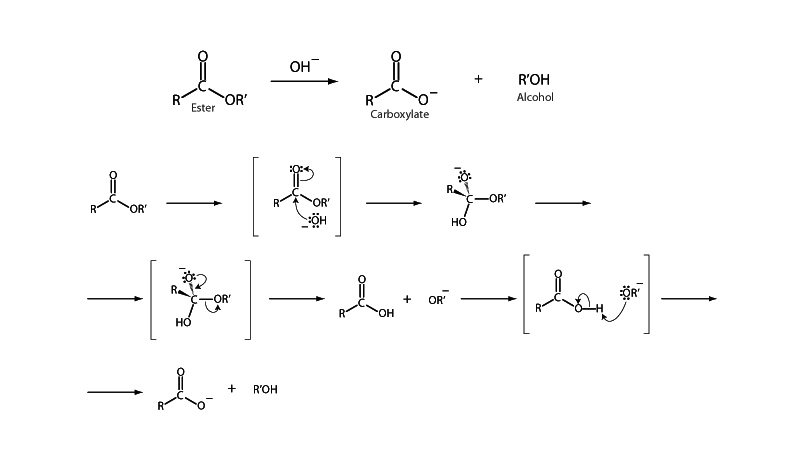
Ester hydrolysis is promoted both by acid and basic conditions. Acid conditions catalytically enhance the reaction with the addition of a proton to the carbonyl oxygen of the ester group. This enhances the reactivity of the carbonyl group to nucleophilic attack by increasing its electron with-drawing character. Acid catalysis helps a subsequent step as well, with the addition of a second proton to increase the facility of the alkoxy portion to leave as an alcohol.
Alternately, basic conditions may also promote ester hydrolysis. Base promoted ester hydrolysis is also called saponification, occurring with the hydroxide anion acting as the nucleophile. Saponification can be used to produce fatty acid salts (soap) from triglyceride.
Soaps are amphipathic, meaning that they are chemical compounds possessing both hydrophilic and hydrophobic moeities. The carboxylate group end of a soap is hydrophilic, while the fatty acid chain end is hydrophobic. Above a critical concentration, long, straight chain carboxylate salts form a colloidal dispersion of spherical aggregates in water. In such micelles, the hydrophilic carboxylate groups localize to the outside and the lipophilic hydrocarbon chains towards the interior.