Interdisciplinary Note (27 of 29)
A major theme with nucleophilic acyl substitution, both in organic chemistry class and on the MCAT, is that some carboxylic acid derivatives are at a lower free energy (more stable) than others. With any discussion of stability involving free energy, it's important to not only analyze the reagent but also the product. The more stable carboxylic acid derivatives are those that produce an unstable leaving group through the reaction. A good rule of thumb is that an unstable leaving group is a strong base. Hence, amides are very stable to acyl substitution because the amide anion leaving group, an extremely strong base, is very unstable. Carboxylic acids and esters are stable, as well, and you can remember that if you remember that hydroxide and alkoxide are strong bases. They are not going to want to leave, thermodynamically speaking, and in biochemistry, there will frequently be an essential residue in the active site, such as a histidine, ready to serve as an acid catalyst and protonate these leaving gropus as they go so that they might leave as water or alcohol, respectively, instead.
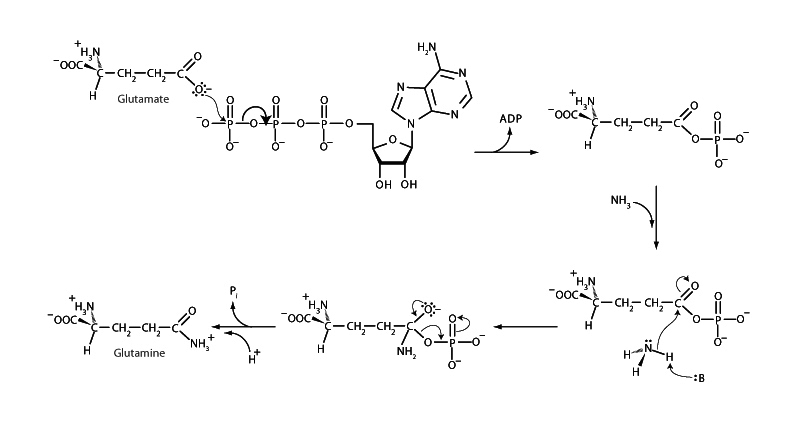
On the other side of things, you might recall it is a major issue in organic chemistry class that acid halides are high energy. Compared to amide, hydroxide, or alkoxide, chloride and bromide are both much better leaving groups. Remembering that acid halides are high energy is a crucial concept in organic class for synthesis questions. If you could form the acid halide, it was a downhill glide to form any of the other carboxylic acid derivatives. This logic is extremely relevant to biochemical synthesis, as well. Acid halides are not present in biochemistry, however. In biochemistry, the role of high energy acyl compound will be played by either a thioester or a phosphate anhydride.
To understand this logic is to understand the personality of Coenzyme A. The bond between coenzyme A and the acyl group it carries is a thioester linkage. The acetyl group on acetyl CoA or the fatty acyl on fatty acyl CoA is primed for nucleophilic acyl substitution. The citrate synthase reaction is driven by hydrolysis of the thioester linkage in acetyl CoA. As another example, in triglyceride synthesis, it is a free energy decrease to form the ester linkages in the triglyceride. Because the fatty acids arrive as fatty acyl CoA, in other words, the esterification reaction with glycerol is spontaneous.
Phosphate anhydrides are also centrally important in this way. Over and over again in biosynthesis, you will see ATP utilize its phosphoryl transfer potential to turn a carboxylate into a phosphate anhydride. This is the same logic from organic class as using thionyl chloride to create an acid halide at the start of bench-top synthesis. Now that the synthesis intermediate is a phosphate anhydride (either with a single phosphate or by addition of AMP). Such a group can now easily become an amide or an ester. When you see this happening, which you will, over and over again, once you start noticing it, you can begin to understand one of the reasons ATP got the job billions of years ago.