Interdisciplinary Note (25 of 29)
The reactions of carboxylic acids and carboxylic acid derivatives represent one of the classes of organic chemistry reactions that play a prominent role in biochemistry. Foremost among these reactions is the nucleophilic acyl substitution mechanism. Nucleophilic acyl substitution appears in one form or another all over the biochemistry from the hydrolysis of triglycerides, serine protease mechanism to histone acetyl transferase. Along with the mechanisms of SN2 substitution and aldol addition, nucleophilic acyl substitution ranks among AAMC's favorite mechanisms.
Acyl transfer is a primary mode of interconversion between carboxylic acid derivatives including carboxylic acids, esters, amides, anhydrides, thioester, etc. The basic pattern for the reaction mechanism is as follows. A nucleophile such as water, hydroxide, alcohol, alkoxide, amine, etc., attacks the carbonyl carbon of the carboxylic acid, ester, anhydride, etc. With the electron pair from the nucleophile forming a bond with the carbonyl carbon, the carbon lets go of the π electrons in its bond to oxygen, so they go up on the oxygen forming an oxianion (unless the oxygen had been previously protonated in acid catalysis, in which case a hydroxyl group forms). Now we have a tetrahedral intermediate. So far, everything is directly analogous to the first step of the nucleophilic additions to aldehydes and ketones. However, in the case of nucleophilic acyl substitution, the tetrahedral intermediate collapses with the electrons on the oxygen returning to the double bond with oxygen, an elimination step resulting in the departure of the group which had characterized the reagent's type of carboxylic acid derivative. If a hydroxide nucleophile attacked an ester, alkoxide leaves resulting in formation of a carboxylic acid. If an amine attacked an acid anhydride, carboxylate leaves resulting in formation of an amide. These two examples need to be as clear as a bell. If you do not have nucleophilic acyl substitution down backwards and forwards, the MCAT is likely to ding you a couple of times.
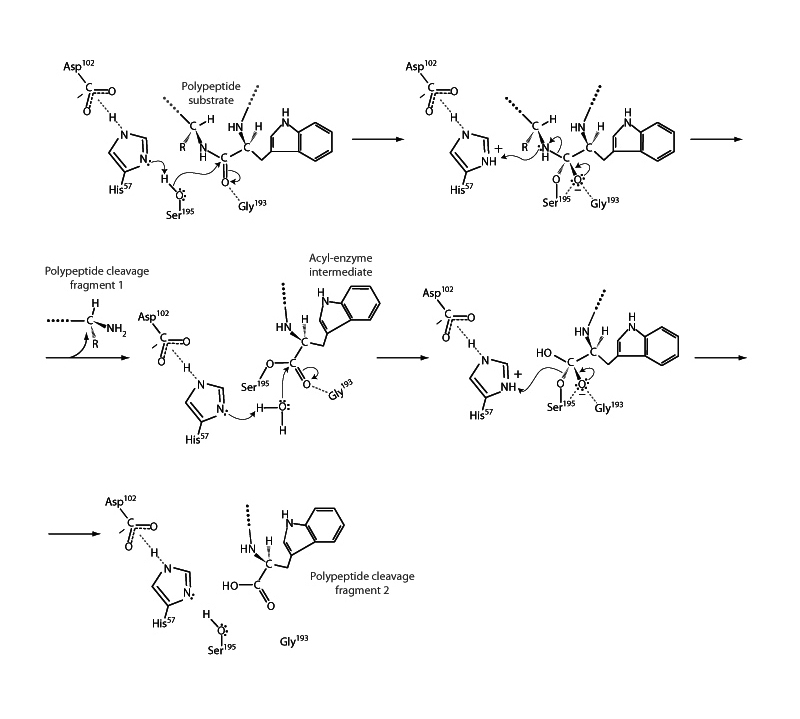
Acyl exchange is one of the most important reaction types in biochemistry. This is exemplified by the mechanism of serine proteases such as chymotrypsin. In the overall mechanism, two acyl exchanges occur. The active site promotes the reactivity of the hydroxyl group of a serine residue to attack the peptide bond, which is an amide linkage. Essential residues in chymotrypsin promote the nucleophilicity of the serine, stabilize the tetrahedral intermediate, and promote the ability of the N-terminus of a departing peptide fragment to leave. Resolution of the intermediate upon the departure of the leaving group (a peptide fragment) forms an acyl enzyme complex. That completes the first acyl substitution. An alcohol (serine) attacked an amide (the peptide bond) to form the tetrahedral intermediate. A peptide fragment leaves with collapse of the intermediate to form an ester between the enzyme serine and the remaining peptide fragment. The second acyl exchange occurs next, which is hydrolysis of the enzyme ester to regenerate the enzyme and release the other enzyme fragment.