Interdisciplinary Note (28 of 29)
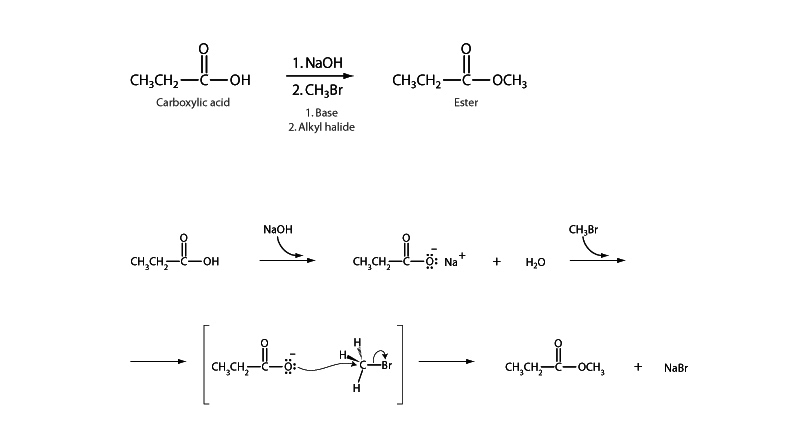
There is a long MCAT tradition of questions about proposed mechanisms for formation of an ester with 18O labeled carboxylic acid reagent. If the ester has been arrived at via nucleophilic acyl substitution, some 18O will be in the solvent, having left during the collapse of the tetrahedral intermediate. (Remember that no matter how they draw it, the two oxygens in a carboxylic acid are equivalent because the proton exchanges 100,000 times a second.)
On the other hand, if the the ester were arrived at via SN2 reaction with carboxylate nucleophile, all of the 18O will still be present in the ester product. There are a million variations of this question. The heart of the question is always about seeing that you understand the mechanism of nucleophilic acyl substitution.