Interdisciplinary Note (29 of 29)
Keto acids are substances with both a carboxyl group and a carbonyl group. In other words, keto acids are carboxylic acids and they are also ketones. Keto acids play a central role in biochemistry.
In α-keto acids, the carbonyl group is located on the carbon adjacent to the carboxyl group. With β-keto acids, the carbonyl group is on the next carbon, the second one away from the carboxyl group.
α-Keto acids have an ancient, intimate connection to the amino acids that build proteins (α-amino acids). The most prominent α-keto acids in biochemistry are the major metabolic crossroads of pyruvate, oxaloacetate, and α-ketoglutarate. As your conception of metabolic integration grows, one of the things you come to appreciate is the central importance of the transaminase interconversion of pyruvate and alanine, oxaloacetate and aspartate, and α-ketoglutarate and glutamate. You come to think of each of these α-keto acids and their corresponding amino acids reached through transaminase as brothers. Many of the other amino acids pass through α-keto acids in their degradation pathways. Not always, but often, the amino acid degradation pathway begins with transaminase removing the nitrogen forming an α-keto acid intermediate with the nitrogen now shuttling towards the urea cycle.
β-keto acids also figure prominently in biochemistry. In the citric acid cycle, for example, there is a β-keto acid, oxalosuccinate, an unstable intermediate in the isocitrate dehydrogenase mechanism. Another important β-keto acid is acetoacetate, which is one of the ketone bodies (ketone bodies are nutrients the liver makes from fatty acids and feeds the body with in the fasted state when glucose is scarce.) β-keto acids are unstable because they decarboxylates easily. β-keto acids are spontaneously labile to decarboxylation. Decarboxylation of a β-keto acid is one of the organic mechanisms on the AAMC MCAT topic list. The gist is that the CO2 (the carboxyl group) will leave easily because what it leaves behind is a resonance stabilized enolate anion. After oxalosuccinate decarboxylates, it forms α-ketoglutarate, so, to summarize the isocitrate dehydroenase mechanism, oxalosuccinate is produced through oxidation of isocitrate by NAD+; oxalosuccinate then spontaneously decarboxylates to form α-ketoglutarate. This is the source of the first CO2 produced by the citric acid cycle. The ketone body acetoacetate also decarboxylates slowly and steadily while it is in the blood, producing acetone, which is also considered a ketone body.
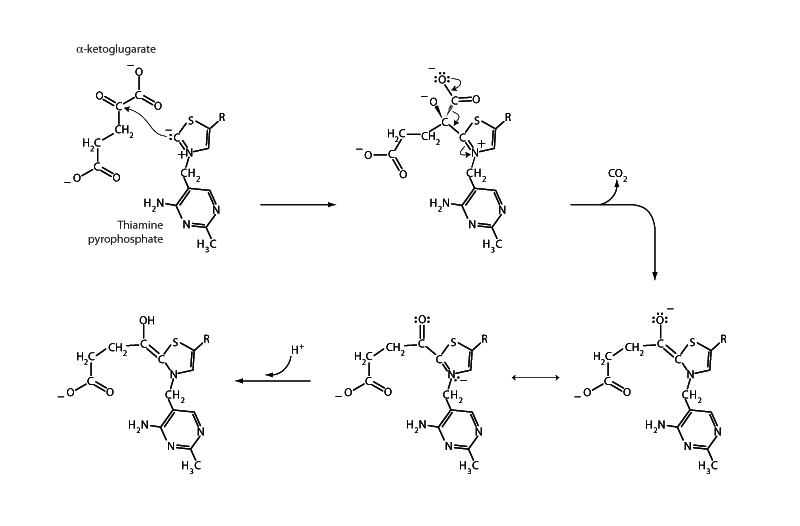
The second CO2 produced in the citric acid cycle is the product of the decarboxylation of α-ketoglutarate in the next step, α-ketoglutarate dehydrogenase. α-Keto acids can also be decarboxylated. However, this is much more difficult to accomplish than decarboxylation of a β-keto acid. Decarboxylation of α-keto acids in biochemistry is one of the big jobs of the coenzyme thiamine pyrophosphate (TPP). TPP decarboxylates pyruvate in pyruvate dehydrogenase, and it decarboxylates α-ketoglutarate in α-ketoglutarate dehydrogenase. It is a figure of merit to be aware that pyruvate dehydrogenase and α-ketoglutarate dehydrogenase are homologous multi-enzyme complexes. In the future, during a particularly brutal week in medical school, you will also learn that TPP carries out a similar decarboxylation during the synthesis of the branched chain amino acids valine, leucine, and isoleucine.