Interdisciplinary Note (24 of 29)
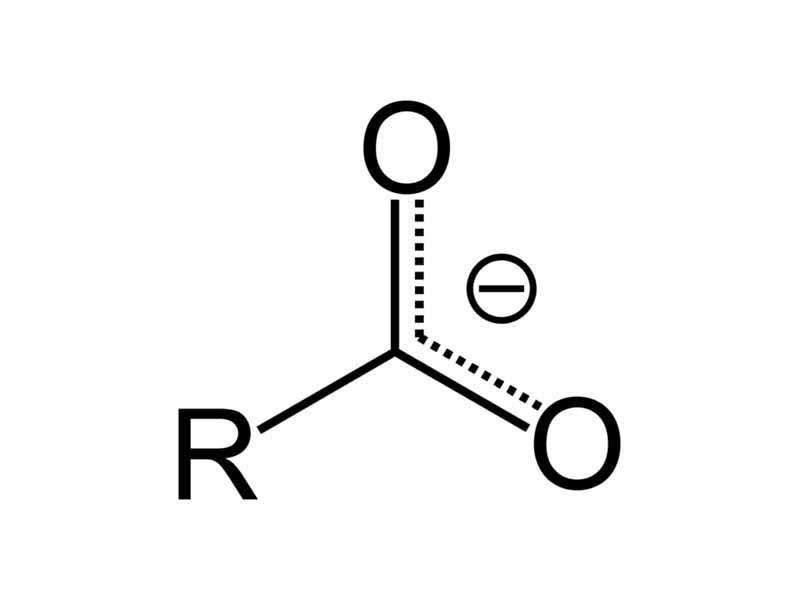
Carboxylic acids are classified as weak acids. Typically, the pKa's of a carboxylic acid is approximately 5. Even though they are categorized as 'weak' acids, having a pKa's greater than 1, carboxylic acids are much stronger acids than alcohols. This is because the product of ionization is a resonance stabilized carboxylate ion, so the acid-base equilibrium is shifted to favor ionization when compared to alcohols.