Interdisciplinary Note (5 of 16)
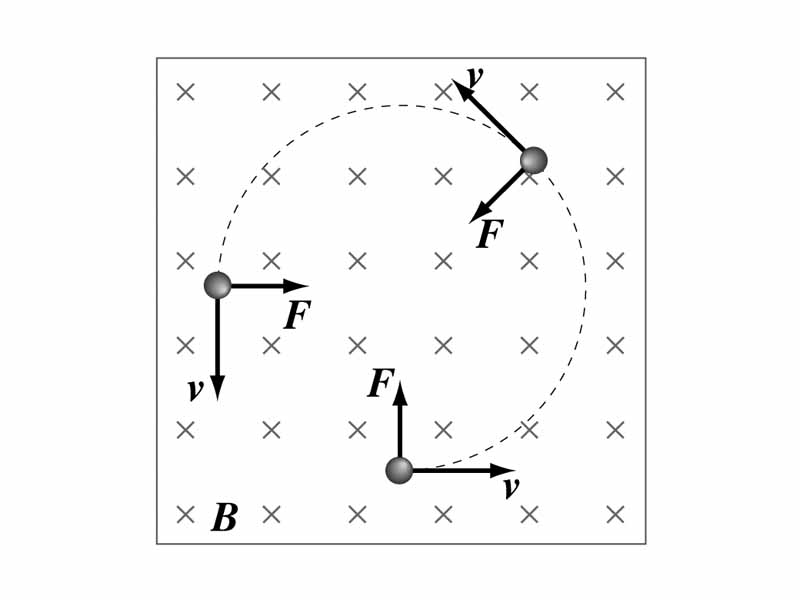
Because the magnetic force on a moving, charged particle is always perpendicular to particle velocity, a steady magnetic field does no work when a particle is displaced. (A corollary is that a magnetic field alone cannot alter the kinetic energy of a particle). Also, if the initial particle velocity is completely perpendicular to the uniform magnetic field into which it is moving, the magnetic force will have the characteristics of centripetal force, and the particle will move in a circle. If there is a component of the particle's velocity parallel in three dimensional space to the magnetic field, the particle will spiral around the field lines.
In the case of a particle moving with uniform circular motion within a magnetic field, the radius of the circle will be greater the greater the initial mass and velocity of the particle, and less depending on the magnitude of particle charge and the strength of the magnetic field. Another way to say this is that with a given speed into a given field, the radius of curvature will depend on the particle's charge to mass ratio. When we reflect on a basic physics concept in MCAT preparation, the concept may not be requiring special attention because of its importance in itself but because of its importance for something else. This particular idea, the dependence on the curvature of the path on charge to mass ratio, is crucial for making the basic function of the common types of apparatus in mass spectroscopy intelligible.
In mass spec, a molecule is ionized, frequently through bombardment by electrons or X-rays (ionizing radiation). The molecule breaks apart to produce a collection of ion fragments. These are accelerated through a voltage to enter a magnetic field. The curvature of the path of a particular ion fragment corresponds to its charge to mass ratio and allows the analytical separation of the fragmentation pattern into a mass spectrum. Mass spectroscopy allows for the identification of molecular mass. The technique also provides structural insights and isotopic data.
So you aren't taken by surprise, we should mention that some mass spectroscopy techniques do not employ a magnetic field to produce the separation by charge to mass ratio. A very significant technique in the protein lab, for example, is Matrix Assisted Laser Desorption Ionization - Time of Flight (MALDI-TOF) mass spec of proteins. In that technique, charge to mass ratio of peptide ions corresponds to their acceleration through a uniform electric field, so they are separated by their time of flight across the space between two capacitor plates.