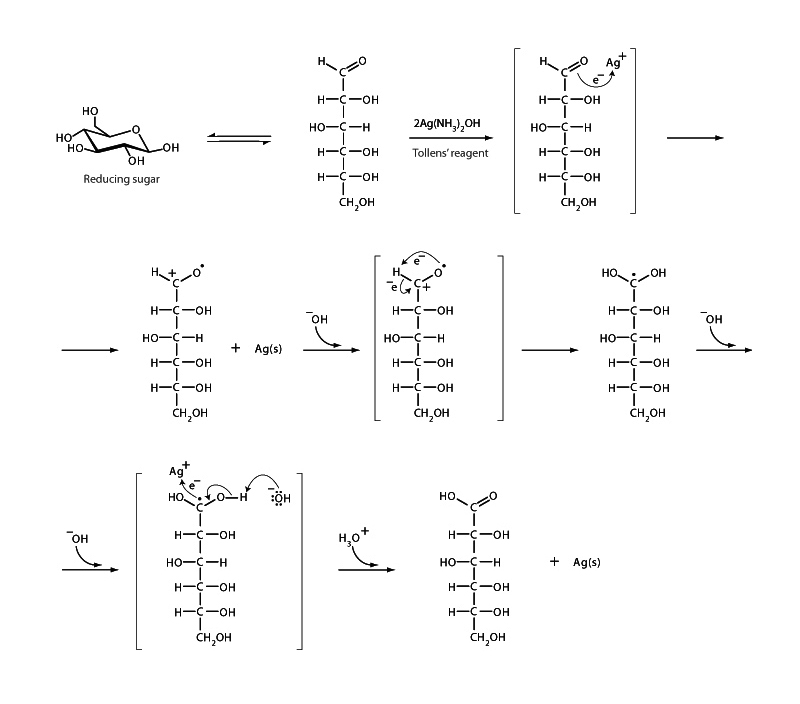
Tollens' test employs a silver ammonia complex [Ag(NH3)2]+ to diagnose the presence of aldehyde. A positive test is indicated by the precipitation of elemental silver producing a characteristic 'silver mirror' on the inner surface of the reaction vessel. Biochemists use Tollens' test to determine the presence of a reducing sugar, a sugar capable of acting as a reducing agent, in the case of Tollens' test, to reduce silver ions. Reducing sugars possess a free aldehyde group. The cyclic hemiacetal forms of aldoses can open to reveal an aldehyde and certain ketoses can undergo tautomerization to become aldoses. Nonreducing disaccharides like sucrose have glycosidic bonds between their anomeric carbons and thus can't convert to an open-chain form. Reducing disaccharides like lactose and maltose have only one of their two anomeric carbons involved in the glycosidic bond, so through the reverse of hemiacetal formation, they can convert to an open-chain form having an aldehyde group.