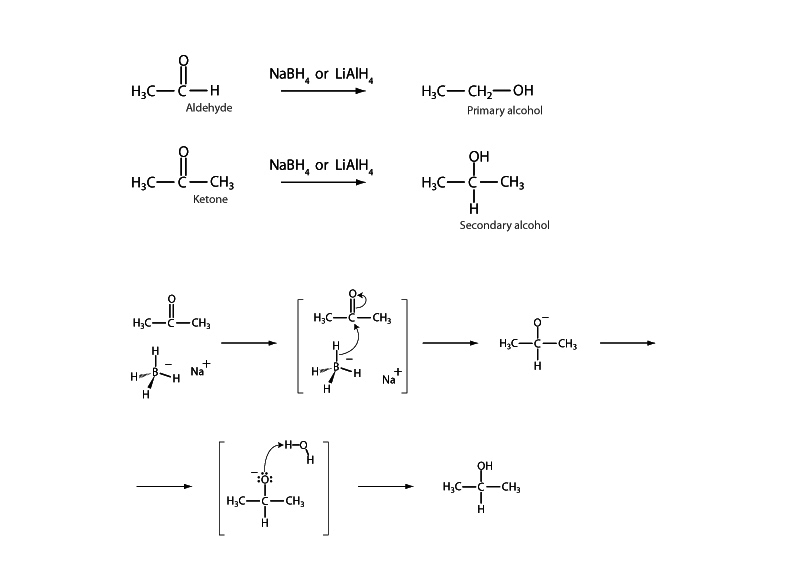
The reducing agents NaBH4 or LiAlH4 transform an aldehyde or a ketone into an alcohol. The reduced form of an aldehyde is a primary alcohol. For a ketone the reduced form is a secondary alcohol. Some reductions, such as Wolff-Kishner or Clemmensen reduction, will reduce aldehydes and ketones all the way to alkanes. Catalytic hydrogenation will reduce a benzylic carbonyl group to an aliphatic carbon as well.
Be sure of the sequence of oxidation states of carbon among the various functional groups. This is extremely important in biochemistry. The progression from more reduced to more highly oxidized forms goes as follows: alkyl, alcohol, aldehyde, ketone, carboxylic
acid, carbon dioxide. A very useful exercise is to build the habit of assigning changes in oxidation state in terms of oxidation numbers when you see movement between these forms through oxidation-reduction. Remember that in assigning oxidation numbers, electron control over shared electrons is attributed to the more electronegative atom. Then you decide whether a particular element has gained or lost electrons in forming the bond. For example, in the reduction of an aldehyde to a primary alcohol shown at left, the oxidation number of the relevant carbon has changed from +1 to –1. For the reduction of the ketone, the oxidation number of the carbon has changed from +2 to 0.