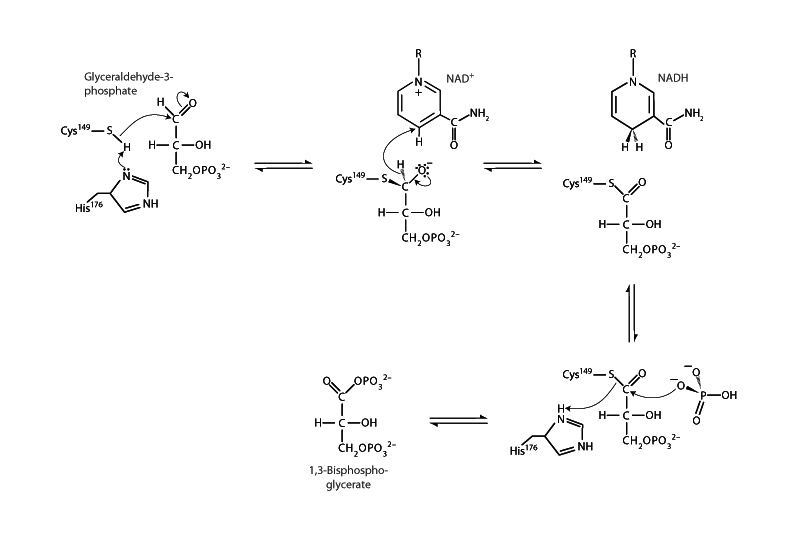
Glyceraldehyde 3-phosphate dehydrogenase, which catalyzes the sixth step of glycolysis, is a fascinating enzyme. The GAPDH mechanism demonstrates a central motif of energy metabolism, which is the coupling of an exergonic oxidation process with the establishment of phosphoryl transfer potential. In transitioning from an aldehyde to a carboxylic acid in a normal redox process, the system would be expected to lose free energy in heat flow to the environment, but here, the transition produces a phosphate anhydride, 1,3-bisphosphoglycerate, which has preserved the redox energy in the form of a compound with a very high phosphoryl transfer potential.
The mechanism starts with a cysteine residue in the active site of GAPDH attacking the carbonyl group of GAP, creating a hemithioacetal intermediate. Next, the coenzyme NAD+ accepts a hydride ion (H-) becoming NADH while oxidizing GAP to a thioester intermediate. Like phosphate anhydrides, thioesters are activated carboxylic acid derivatives. This is how the redox energy is preserved. Thioesters are much higher in energy than the carboxylic acid species that would have resulted from the simple oxida- tion of an aldehyde. Finally, a molecule of inorganic phosphate attacks the thioester forming a tetrahedral intermediate, which then collapses through the acyl substitution addition-elimination pathway becoming 1,3-bisphosphoglycerate while releasing the thiol group of the enzyme's cysteine residue.