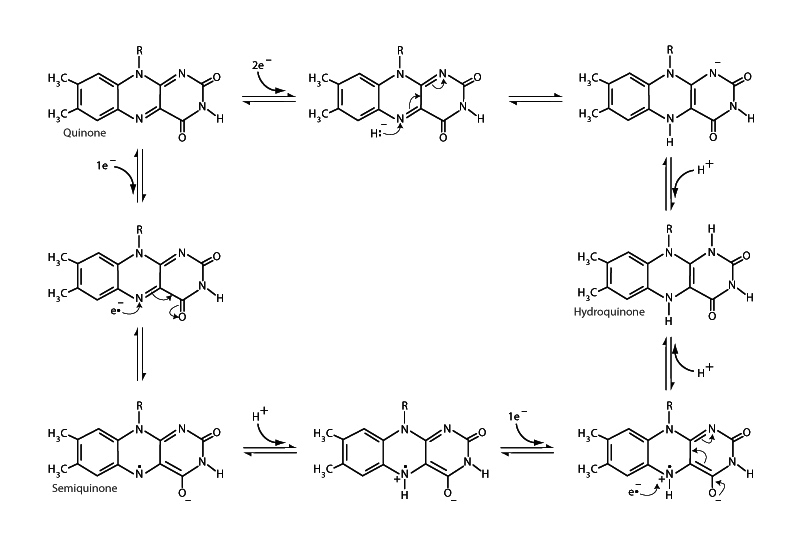
A flavoprotein contains a flavin moiety in the form of one of the prosthetic groups flavin adenine dinucleotide (FAD) or flavin mononucleotide (FMN). Important flavoproteins in energy metabolism include succinate dehydrogenase, &alpha-ketoglutarate dehydrogenase, pyruvate dehydrogenase and acyl-CoA dehydrogenase. There are many flavoproteins in metabolism. Humans have 90 flavoprotein encoded genes. Unlike the other redox cofactors in metabolism, such as NADH, which transfers electrons two at a time, or Coenzyme Q10, which transfers electrons 1 at a time, flavins are capable of carrying out either 1 or 2 electron transfers. Represented in the clock-wise path from the quinone above left, FAD can accept 2 electrons and 2 protons to become FADH2 (hydroquinone form). Alternatively, the free radical semiquinone (FADH·) can be formed by a single electron reduction of FAD, as shown in the counter-clockwise path, followed by another single electron transfer to form FADH2. Both pathways are reversible, so FADH2 can also donate 1 or 2 electrons.
The flavin moeity has a more positive reduction potential than the nicotinamide ring of NAD+. Flavin is a strong oxidizing agent. The cell utilizes its high reduction potential in many energetically difficult oxidation reactions such as dehydrogenation of a C-C bond to an alkene.