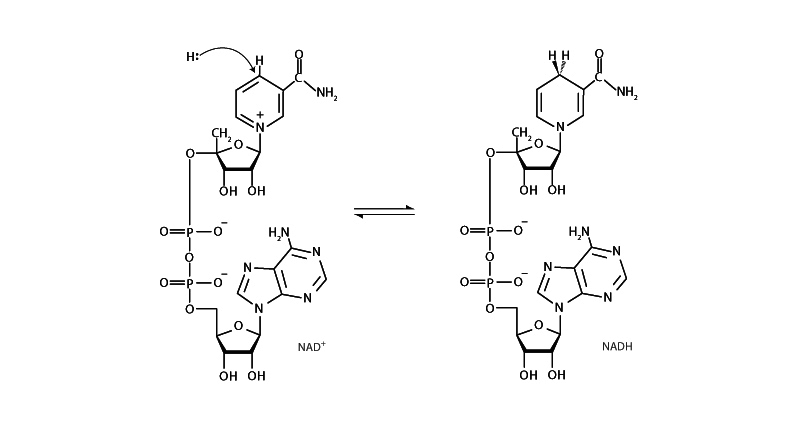
An essential coenzyme of many oxidoreductases, NADH (nicotinamide adenine dinucleotide) is the primary carrier of reducing equivalents for energy metabolism, carrying electrons from one redox reaction to another. NAD+ is an oxidizing agent, accepting electrons from other molecules, forming NADH, which can then be used as a reducing agent. Another coenzyme, NADPH, differs from NADH in having a phosphate group on C-2 carbon of its adenosine ribose. NADPH performs chemically in virtually the same way as NADH. NADPH is the primary source of reducing equivalents for biosynthesis.
Nicotinamide adenine dinucleotide is a hydride (H-) donor and receiver. Reactivity is restricted to the nicotinamide portion of the molecule. When NAD+ receives a hydride to form NADH, the nicotinamide ring loses aromaticity. The electrons are kept in an elevated energy. This is why NADH is such a good electron transfer agent. When it donates its electrons it regains aromaticity.
Note that the C-4 carbon of nicotinamide is prochiral. Using deuterium labeling it has been possible to distinguish the direction of hydride approach for many oxidoreductase reactions. This tells us how the nicotinamide ring of the coenzyme is positioned in the active site vis-a-vis the substrate. Depending on the enzyme, the hydride donor will be positioned either above or below the plane of the C4 carbon and donate to either the “re” face of NAD+ or the "si" face, respectively.