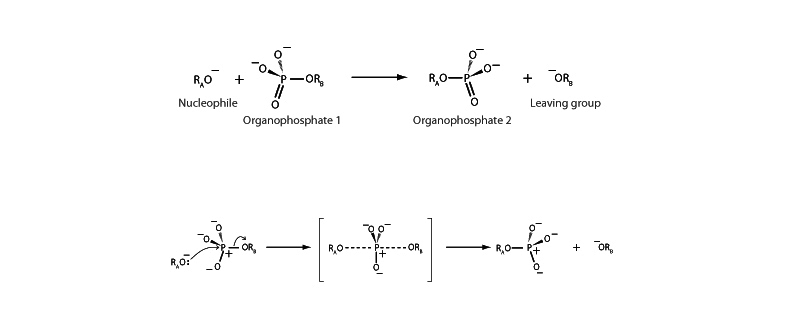
Because phosphate esters play a central role in biochemistry, phosphoryl transfer reactions are correspondingly important. The study of chiral phosphate derivatives has demonstrated that several mechanistic variants of the phosphoryl transfer reaction occur. In the most common variant, the in-line mechanism shown above, the phosphoryl transfer reaction occurs along a pathway that is very similar to an SN2 reaction at a carbon, but across a phosphorus atom instead. In the in-line phosphoryl transfer mechanism, the nucleophile approaches the phosphorus from the backside, opposite the leaving group. As the nucleophile begins to attach and the leaving group begins its departure, the bonding geometry at the phosphorus atom changes from the tetravalent reagent to a pentavalent transition state. As in SN2, the stereochemistry of the substituent groups flips when the leaving group departs and there is an inversion of configuration.
Note that this in-line SN2-type mechanism is not the only possible mechanism of phosphoryl transfer. A dissociative mechanism may also occur, similar to the SN1 reaction in which elimination of the leaving group occurs first, forming an unstable intermediate which then captures the nucleophile. There is also a concerted variation that involves a pentavalent transition state which forms by nucleophilic attack from the same side as the leaving group.