When the body is in the fasted state, oxaloacetate becomes in short supply in the liver because it is being used to form glucose through the process of gluconeogenesis. Oxaloacetate is thus unavailable to bind with acetyl CoA. Additionally, the high level of acetyl CoA present in the cell inhibits the pyruvate dehydrogenase complex, whereas pyruvate carboxylase becomes activated for gluconeogenesis. High levels of ATP and NADH inhibit the enzymes citrate synthase and isocitrate dehydrogenase in the TCA cycle. Under these conditions acetyl CoA is diverted into the formation of ketone bodies, which are water-soluble transportable forms of acetyl units and which serve as a perfectly acceptable fuel for most of the needs of peripheral tissues. The three ketone bodies are acetoacetate, β-hydroxybutyrate, and acetone.
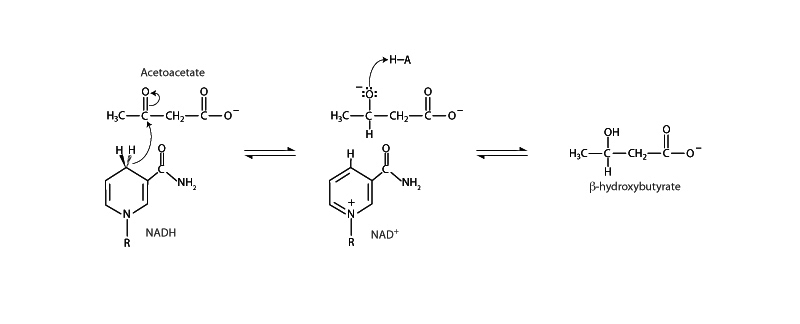
β-hydroxybutyrate is formed by the reduction of acetoacetate by the enzyme β-hydroxybutyrate dehydrogenase. This activity allows the liver to dispose of some of the surplus hydrogen that accumulates during β-oxidation of fats. β-hydroxybutyrate dehydrogenase employs NADH in its typical role as a hydride donor, reducing the carbonyl group of acetoacetate to a secondary alcohol. The activity of β-hydroxybutyrate dehydrogenase depends on the NADH/NAD+ ratio within hepatocyte mitochondria.