Gluconeogenesis begins in the mitochondria with the pyruvate carboxylase reaction, which carries out the formation of oxaloacetate by the carboxylation of pyruvate. In the next step of gluconeogenesis, oxaloacetate will be decarboxylated and phosphorylated by PEP carboxykinase to form phosphoenolpyruvate. Utilizing ATP and GTP, respectively, these two reac- tions push the substrate up the energy hill, part of the particular reactions, along with fructose bispho- sphatase and glucose 6-phosphatase that sidestep the exergonic reactions of glycolysis on the reverse glucogenic path.
Pyruvate carboxylase is an important control point for the gluconeogenic pathway. The enzyme is stimulated by high levels of acetyl-CoA (the signal of β-oxidation of fatty acids) and inhibited by high levels of ADP and glucose.
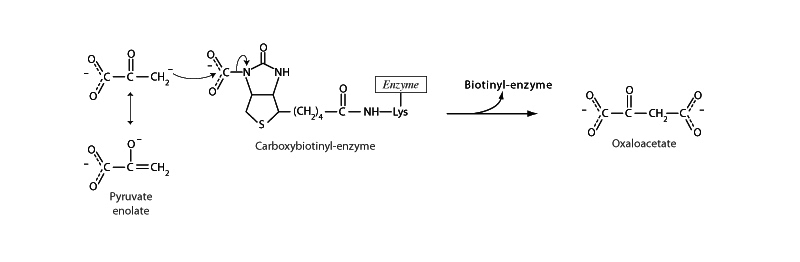
Pyruvate carboxylase relies on a covalently attached prosthetic group, biotin. Biotin serves as a carrier of activated (high energy) CO2, which it obtains from another active site on the enzyme, the ATP-bicarbonate site. Carboxylation of biotin is the process requiring ATP. An interesting feature of this prosthetic group is that the long chain holding biotin to its enzyme allows it to swing between active sites. In this way biotin is like lipoamide in the pyruvate dehydrogenase complex.
After activation, the carboxy-biotin swings over to the pyruvate site of the enzyme, and the enol form of pyruvate attacks it. This mechanism illustrates a key feature of the reactivity of aldehydes and ketones in biochemistry, an aspect you will see over and over again. The α carbon of an enol formed through keto-enol tautomerism is nucleophilic. The nucleophilicity of α carbons is a fundamentally important aspect of the reactivity of aldehydes and ketones. We will see more of this behavior in the reactions based on aldol addition. We will also see something similar in the reactions that rely on imine-enamine tautomerism.