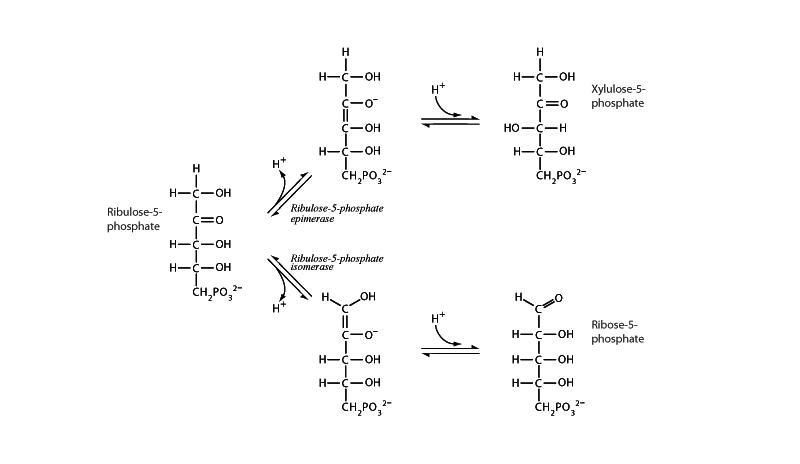
The pathway branches from ribulose-5-phosphate to begin the non-oxidative phase of the pentose phos- phate pathway with keto-enol tautomerism the essential mechanism of both branches. Ribulose- 5-phosphate 3-epimerase produces an epimer of ribulose-5-phosphate (An epimer is a kind of diastereomer where the difference is at only one chiral center). The other branch, ribulose-5-phosphate isomerase, transforms the aldose ribulose-5-phosphate into the ketose ribose-5-phosphate.
After ribulose-5-phosphate 3-epimerase catalyzes transformation of the substrate into the enol form, it exerts a stereospecific influence on the equilibrium conversion back to the ketone, so that when the ketone reforms, it comes back with an inversion of configuration at its number 3 carbon. This illustrates an essential point in biochemistry. Enzyme catalysis takes place within three dimensional active sites with multiple points of interaction with the substrate, so stereospecific results can be achieved far beyond what is generally possible for benchtop organic chemistry.
The ribulose-5-phosphate isomerase reaction is very similar to the triose phosphate isomerase mechanism, employing a common enol tautomer to move between ketose and aldose forms.