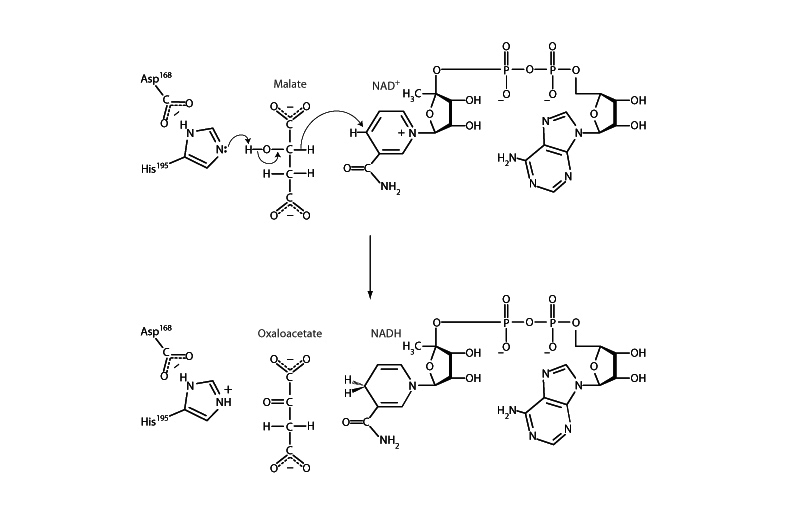
In the citric acid cycle, malate dehydrogenase reversibly catalyzes the oxidation of malate to oxaloacetate with reduction of NAD+ to NADH. Malate dehydrogenase also plays a role in gluconeogenesis, although it is not in the net pathway. In gluconeogenesis, pyruvate carboxylase forms oxaloacetate from pyruvate in the mitochondria. Malate dehydrogenase reduces the oxaloacetate to malate so that it can traverse the inner mitochondrial membrane. Once in the cytosol, the malate is oxidized back to oxaloacetate by a cytosolic form of malate dehydrogenase so that gluconeogenesis can resume with the PEP carboxykinase step.
The malate dehydrogenase mechanism illustrates a recurrent theme in energy metabolism, the oxidation of an alcohol to a carbonyl group by NAD+. Such
reactions involve the removal of two hydrogen atoms from the reactant in the form of a hydride ion (H-), and a proton (H+). The proton is received by a histidine residue (ultimately released to the solution) while NAD+ is reduced to NADH by transfer of the hydride to the nicotinamide ring. Note that when the NAD+ is reduced, the nicotinamide ring loses its aromaticity. This is why NADH such a powerful reducing agent. When it donates these electrons to NADH:ubiquinone oxidoreductase (Complex I) later, the nicotinamide ring will regain its aromaticity.