Substitution reactions upon an alcohol substrate involve replacement of the hydroxyl group with a new functional group. However, hydroxide is a strong base, making hydroxyl groups poor leaving groups. For this reason substitution reactions of alcohols are generally acid catalyzed. Acid catalysis makes it possible for the hydroxyl group to leave as water.
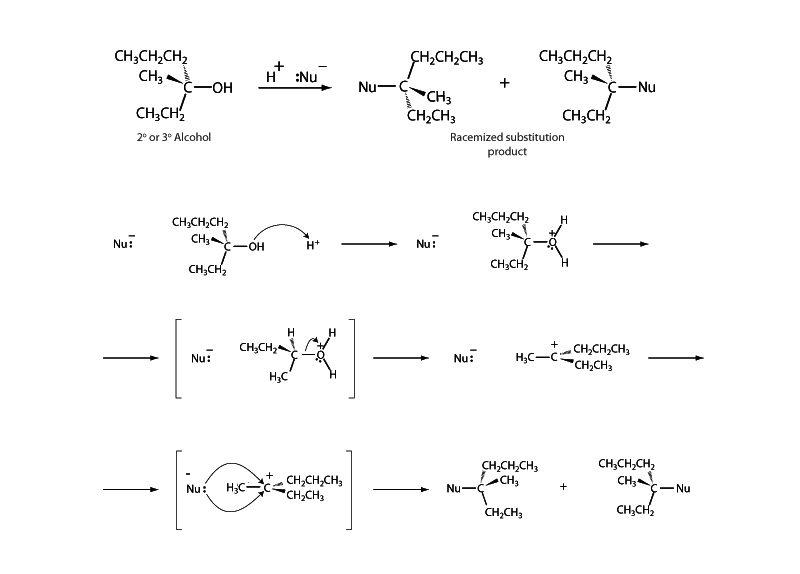
Upon acid catalysis, the SN1 variation of alcohol substitution begins with departure of water and formation of a carbocation. The "1" in SN1 refers to the fact that only the substrate appears in the rate determining step in the SN1 mechanism, carbocation formation, and thus the rate expression is first order. Because the reaction passes through a carbocation intermediate, the SN1 mechanism is much more likely for tertiary alcohols than primary alcohols, where SN2 substitution predominates. Tertiary carbocations are significantly more stable than primary carbocations. Also, remember that rearrangement might occur within a carbocation intermediate if a methyl or hydride shift transforming a secondary into a tertiary carbocation is possible.
The carbocation intermediate is trigonal planar and attack by the nucleophile can occur on either face of the carbocation. This means that even if the reagent is a pure enantiomer, the SN1 mechanism leads at least to partial racemization.