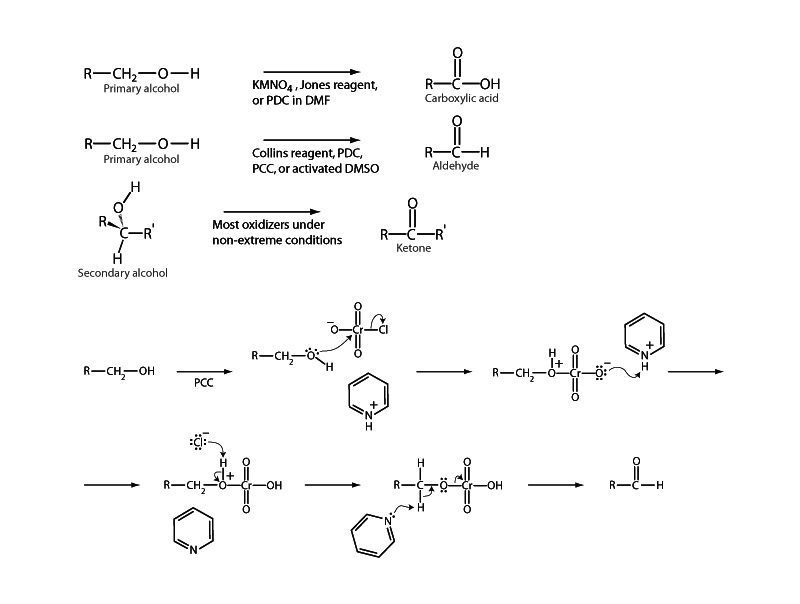
Primary alcohols can be oxidized to aldehydes or to carboxylic acids. In aqueous media, the carboxylic acid is usually the major product. The oxidation of secondary alcohols normally terminates at the ketone stage.
The figure above depicts the mechanism of the oxidation of a primary alcohol by pyridinium chlorochromate (PCC). In the first step the alcohol forms a chromate ester. This places electron withdrawing pressure on oxygen. Oxygen is the second most electronegative element after fluorine. Here oxygen now finds itself in a tug-of-war across chromium with three other oxygen atoms. When pyridinium abstracts a proton from the carbon bonded to oxygen, this solves the problem, because electrons that had been under the control of carbon are free now to shift to oxygen. Oxygen would rather own these electrons than be in a tug-of-war over the electrons it owns in redox terms in its bond with the chromium atom that is in a +6 oxidation state, so it leaves those electrons to become the property chromium. Carbon loses and chromium gains. Oxidation occurs as the carbonyl group forms and the Cr species leaves with an electron pair. In this reaction carbon has been oxidized from a –1 to +1 oxidation state and chromium has been reduced from a +6 to a +4 oxidation state.