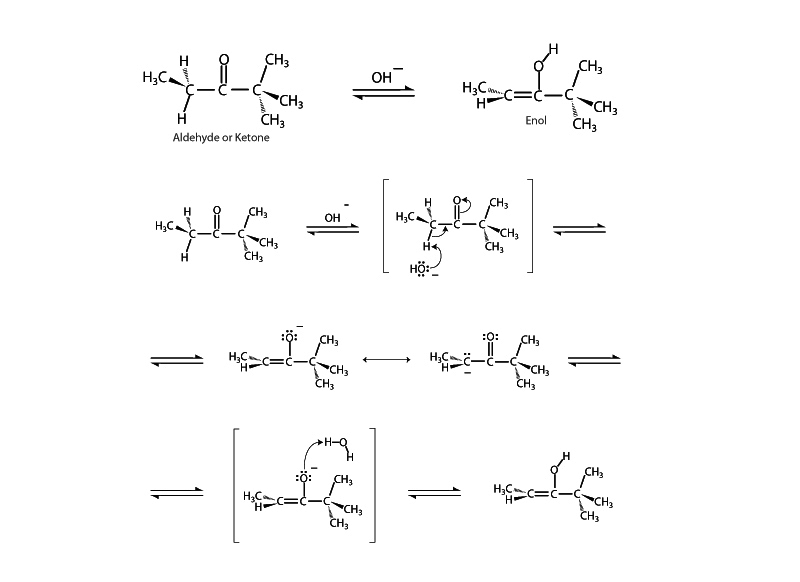
Keto-enol tautomerism is an important factor in the reactivity of aldehydes and ketones. Keto-enol tautomerism can occur with carbonyl compounds if the carbon adjacent to the carbonyl, the α carbon, possesses a hydrogen. Such α hydrogens are acidic. The conjugate base formed is resonance stabilized. The proton departs and electrons shift to form a carbon- carbon double bond with another electron pair moving by conjugation up onto the oxygen. The carbonyl group becomes an alkoxide anion and then captures a proton to become a hydroxyl group. The net process is the transfer of a proton from the α carbon to the carbonyl oxygen accompanied by the shifting of electrons up toward oxygen. The vinylic alcohol formed is referred to as an enol.
A ketone with α hydrogens at two positions has two possible tautomers. Enolization toward the less substituted position will generally be advantaged kinetically (lower activation energy) while enolization towards an interior position will be advantaged thermodynamically (lower free energy). At low temperature, kinetic control prevails and the less substituted double bond is the main reaction product. At high temperature after long reaction times, chemical equilibrium can assert itself and the thermodynamically more stable interior double bond is formed.