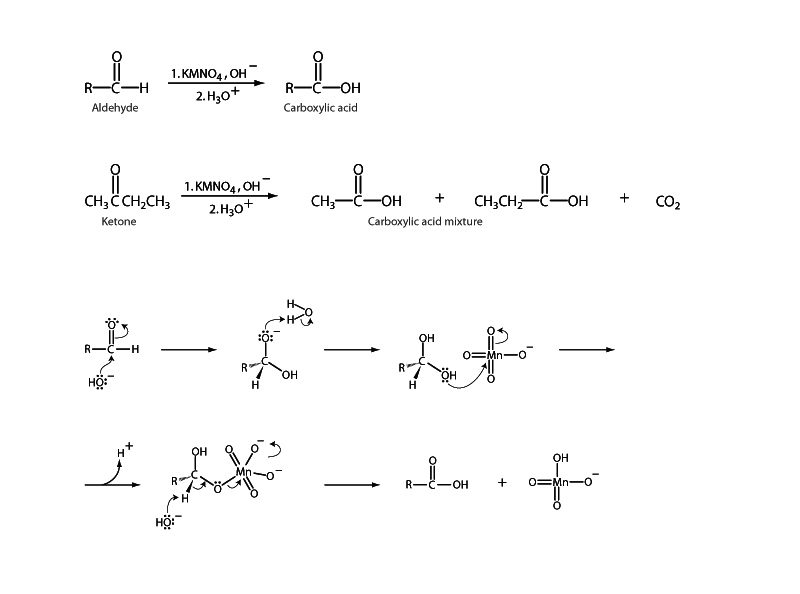
Many of the stronger oxidizing agents such as KMnO4 will transform aldehydes into carboxylic acids. Tol- lens' reagent [Ag(NH3)2]+ is one such oxidant. A shiny mirror of metallic silver is deposited through oxidation of aldehydes by Tollens' reagent, so it is a frequently used test for aldehydes in qualitative analysis. Aldehydes are themselves oxidation products of alcohols.
Be cognizant of the spectrum of oxidation states for organic carbon-oxygen functional groups, beginning with alcohols, which are more highly reduced than aldehydes or ketones. Aldehydes and ketones are in turn more reduced than carboxylic acids and carboxylic acid derivatives.
A strong oxidizing agent like KMnO4 will oxidize a primary alcohol past the aldehyde and up to the carboxylic acid oxidation state, while other, weaker oxidizing agents, like PCC, can be used to form aldehydes from alcohols, not proceeding to oxidize the aldehyde further. In general, normal ketones are not oxidized except under extreme conditions. At high temperature, ketones are cleavage oxidized by a strong oxidizing agent like KMnO4. An exception is a benzylic carbonyl group, which KMnO4 oxidizes easily.