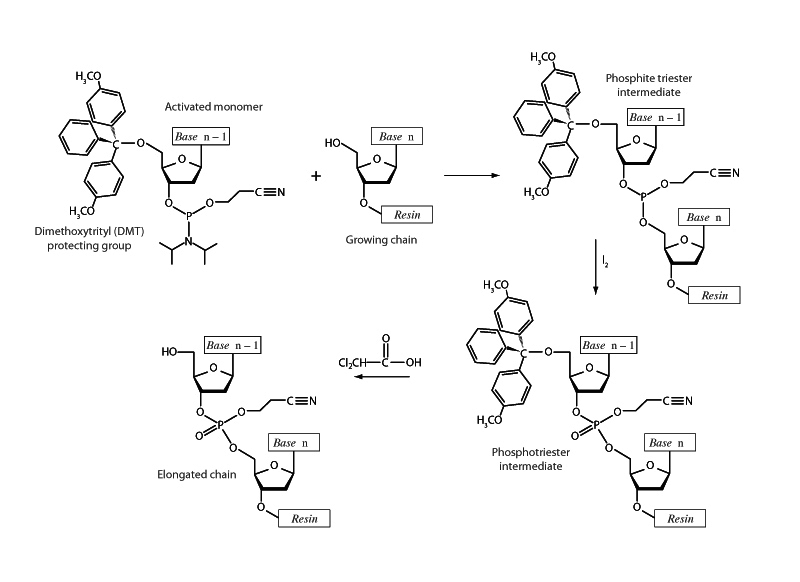
The dimethoxytrityl (DMT) protecting group is widely used for protection of 5'-hydroxy group in nucleosides, particularly in oligonucleotide synthesis, such as in the phosphite triester method of solid phase DNA synthesis shown above. The 5'-hydroxy group of the monomer to be added has been rendered unreactive (protected) by attaching DMT, preventing undesirable side reactions. Upon the addition of the monomer, the DMT will be removed with weak acid.
The gist of the phosphite triester method is that naturally occurring nucleotide phosphate esters are insufficiently reactive to afford convenient synthetic preparation of oligonucleotides in high yields. Using a phosphoramidite derivative dramatically improves the selectivity and the rate of the formation of inter- nucleosidic linkages.
While not specific MCAT content, solid phase DNA synthesis is indicative of the type of procedure the test-writers are apt to choose as the subject matter of a passage. Difficult passages are almost never about whether you have seen the particulars before but about whether you can break things down in terms of the fundamental principles you already know.