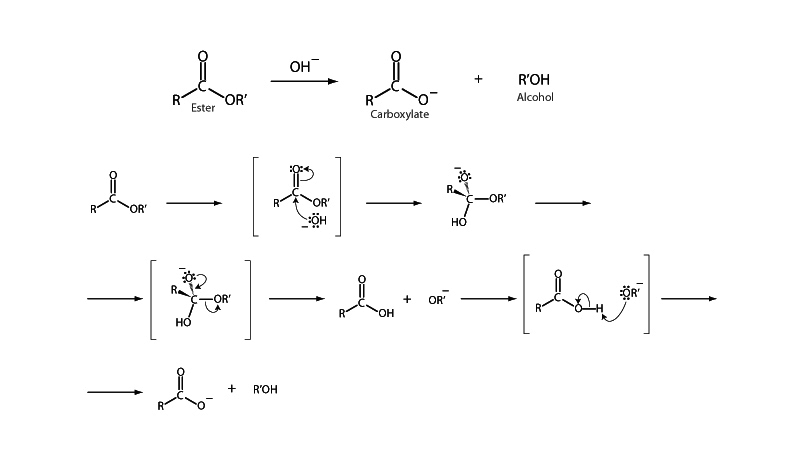
Hydrolysis of an ester under basic conditions is called saponification because it is the chemical reaction underly- ing the traditional method of making soap from glycerol triesters. In saponification, a hydroxide anion adds to the carbonyl group of the ester to form a tetrahedral intermediate. The intermediate then collapses as alkoxide anion departs from the ester. A carboxylic acid forms, which is soon deprotonated by the alkoxide anion. (Note that ester hydrolysis can also be promoted by acid catalysis). Saponification of an ester is an example of an acyl substitution mechanism. In these reactions, attack of the nucleophile forms a reactive intermediate. The tetrahedral intermediate collapses. The carbon-oxygen double bond re-forms, and the original acyl X group is expelled. This is how carboxylic acid derivatives are changed from one type to another.
As a general rule the weaker the basicity of the leaving group of a carboxylic acid derivative, the more reactive it is for acyl substitution. Remember the order of reactivity below, and take special note that thioster and acyl phosphate are the most reactive (highest energy) biologically important forms of those shown. In biochemistry, thioesters and acyl phosphates are your 'activated carboxylates.'