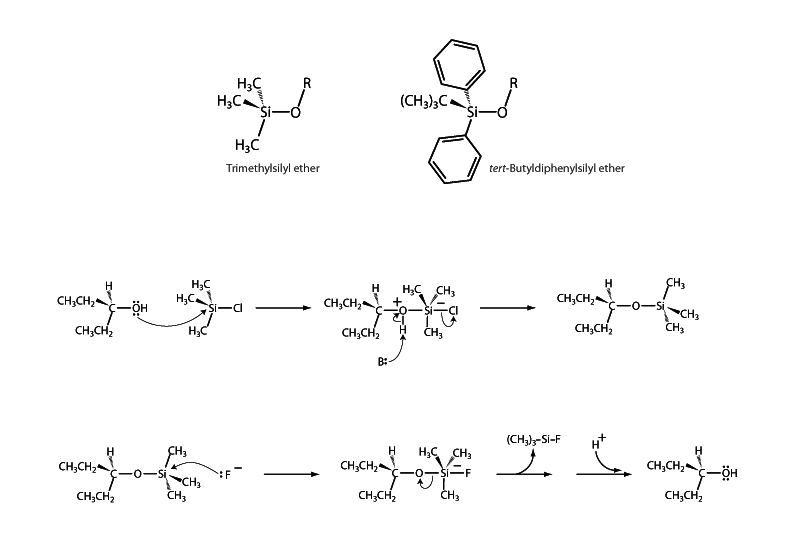
Silylating agents are used in synthetic organic chemistry to protect hydroxyl groups from adverse reaction conditions. In the role of protection, they group forms a silyl ether with the substrate. Silyl groups are particularly useful for this purpose because they can be installed and removed very selectively under mild conditions. Common silyl protecting groups include trimethylsilyl (TMS) and tert-butyldiphenylsilyl (TBDPS).
There are some interesting aspects regarding the use of silyl protecting groups. For example, the bulkier silyl protecting groups, such as TBDPS, will selectively protect primary alcohols in the presence of secondary and tertiary alcohols. Additionally, silyl groups are generally created with at least two of the R groups on silicon being the same. Otherwise, the silicon of the silyl group would be a chiral center. Protection using a racemic mixture of such a silylating agent could create a new stereogenic center and formation of a mixture of diastereomers which might complicate downstream handling.