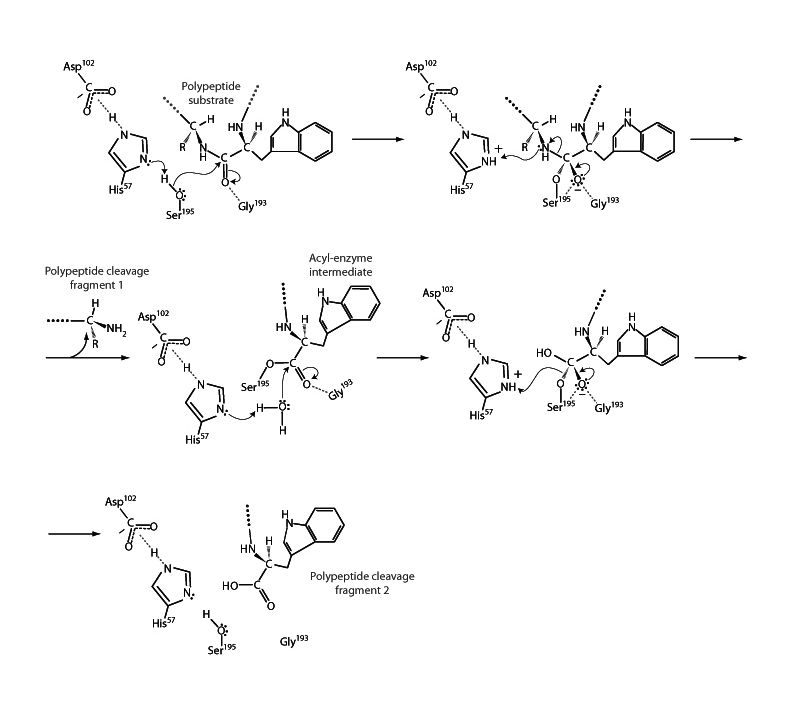
Chymotrypsin is a serine protease. It employs a particularly reactive serine residue to cleave a polypeptide. The chymotrypsin mechanism is one of the classics of enzyme mechanics. Acyl substitution occurs twice in the mechanism. First, the serine residue attacks an amide peptide linkage shared on the N-terminus side by a large nonpolar residue that fits in chymotrypsin's specificity pocket. Serine is assisted through charge relay by a nearby histidine residue and a further aspartate residue, which heighten the nucleophilicity of serine's hydroxyl group by involving it in a particularly strong hydrogen bond. When the tetrahedral intermediate forms, another pocket in the enzyme, the oxyanion hole, stabilizes the negatively charged oxygen atom. Then the intermediate collapses along the acyl substitution pathway, and chymotrypsin releases the C-terminal fragment of the polypeptide with the N-terminal fragment remaining bound to serine by an ester linkage. This serine-peptide ester is next hydrolyzed in the enzyme deacylation step, another acyl substitution with water as the nucleophile.