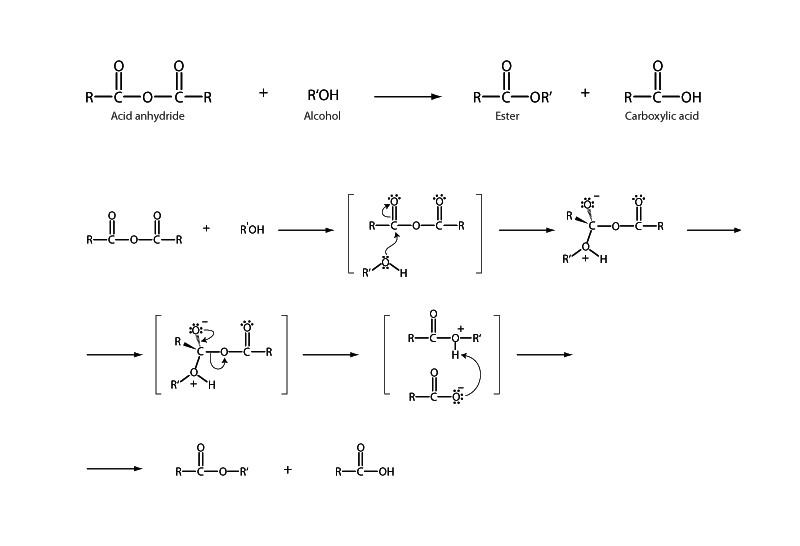
Acid anhydrides are on the unstable end of the spectrum of carboxylic acid derivatives. Like acyl halides, acyl phosphates, and thioesters, acid anhydrides may be readily hydrolyzed, aminolyzed, or esterified through the acyl substitution pathway.
In the case of reaction of an acid anhydride with an alcohol, the product is an ester with a carboxylate anion as the leaving group. Attack of the alcohol forms a tetrahedral intermediate with the anhydride. The tetrahedral intermediate collapses. The carbon-oxygen double bond re-forms, and the carboxylate anion is expelled. The tetrahedral intermediate favors collapse in this direction, instead of expelling the recently joined alkoxide because carboxylate anions are more stable in solution than alkoxide. Just as carboxylate is the weaker base, it's also the better leaving group. Because carboxylate is a more stable anion than alkoxide, acid anhydrides are more reactive than esters.